Research Article
Ann Surg Perioper Care. 2024 ; 9(2) : 1065.
Heated Intraperitoneal Chemotherapy after PRODIGE-7: An Institutional Experience
Fasih Ali Ahmed¹; Malaak Saadah²; Hanna Kakish³; Luke Rothermel³; Richard Hoehn³; John Ammori³*
¹Department of Surgery, University of Pennsylvania, Philadelphia, PA, USA
²Case Western Reserve University School of Medicine, Cleveland, OH, USA
³Division of surgical oncology, University Hospitals Cleveland Medical Center, Cleveland, OH, USA
*Corresponding author: John Ammori, MD Division of surgical oncology, University Hospitals Cleveland Medical Center, Cleveland, OH, 11100 Euclid Avenue, Lakeside 7, USA. Email: John.Ammori@uhhospitals.org
Received: February 26, 2024 Accepted: April 03, 2024 Published: April 10, 2024
Abstract
Introduction: Cytoreductive Surgery (CRS) with Heated Intraperitoneal Chemotherapy (HIPEC) is utilized for selected patients with peritoneal surface malignancies. Following the PRODIGE-7 trial in 2018, CRS without HIPEC for colorectal carcinomatosis was proposed as equivalent in terms of outcomes without the adverse effects associated HIPEC. We examined the practice pattern and post-operative outcomes before and after the results of PRODIGE-7 at a single academic center.
Method: We reviewed all patients who underwent CRS with or without HIPEC by our surgical oncology team between 2011 and 2022. Cases were grouped into two time periods (1:2011-2018, 2:2019-2022) before and after the results of PRODIGE-7 trial. Primary outcome of interest was the change in utilization of HIPEC with secondary outcomes of post-operative morbidity, readmission, and mortality within 90 days of surgery.
Results: A total of 88 patients were included in this analysis; 47 (55%) and 25 (29%) of whom had peritoneal metastases of appendiceal and colorectal origin, respectively. The median Peritoneal Carcinomatosis Index (PCI) was 10 (interquartile range (IQR): 4-18.5) and Completeness of Cytoreduction score was 0. 42 (48%) patients experienced morbidity within 90 days of CRS, most commonly gastrointestinal complications (16, 18%). The median length of stay was 8 days and there were 16 (18%) 90-day readmissions and 2 (2%) mortalities. 44 (90%) and 18 (56%) patients received HIPEC before and after PRODIGE-7, respectively. Their median PCI in time periods 1 and 2 were 8 (IQR: 3-17) and 13 (IQR: 3-19), respectively. There was a reduction in the use of HIPEC among patients requiring major gastrointestinal (GI) visceral resections after 2018. Among patients who underwent colectomy, HIPEC was only used in 6 (26%) compared to 25 (57%) before 2018. On multivariable analysis, postoperative adverse events were more likely for patients with PCI of 20+ (OR:13.62, 95% CI=2.09-88.57, p=0.006) and less likely for patients treated after PROIDGE-7 (OR:0.28, 95% CI=0.08-0.98, p=0.047) and underwent HIPEC (OR:0.17, 95% CI=0.04-0.72, p=0.016).
Conclusion: The results of PRODIGE-7 impacted patterns of practice of treating peritoneal surface metastases with reduction in the use of HIPEC among patients with higher PCI and those requiring major GI visceral resections.
Introduction
Peritoneal Metastasis (PM) is associated with cancer of the gastrointestinal, reproductive, and genitourinary tracts, with Colorectal Cancer (CRC) patients presenting with the most numbers due to the high incidence of this cancer [1]. Cytoreductive Surgery (CRS) for CRC is associated with improved survival for well-selected patients [2]. Heated Intraperitoneal Chemotherapy (HIPEC) is commonly used in combination with CRS for these patients [3]. Studies have demonstrated improved survival with this combination for patients with PM when compared to systemic chemotherapy alone [3]. However, the role of HIPEC in this combination therapy and the proportion of benefit it confers is an unanswered question. HIPEC is associated with adverse effects which have been shown to significantly impact morbidity and mortality [4]. A multicenter study from four French speaking countries demonstrated reduced morbidity and mortality only when patients with less extensive disease (peritoneal carcinomatosis index <20) were treated using CRS-HIPEC. Thus, careful selection of candidates for HIPEC is required for optimal outcomes.
In the last few years, several randomized control trials have been conducted to evaluate the role of HIPEC in the treatment of CRC with PM. The PRODIGE-7 study compared survival of PM from CRC patients treated with CRS alone versus CRS-HIPEC [5]. The results of the PRODIGE-7 study failed to show improvement in overall survival among patients treated with CRS-HIPEC [5]. Next, PROPHYLOCHIP-PRODIGE-15 trial investigated the survival benefit of second look surgery plus HIPEC when compared to surveillance alone among patients with resected disease and high risk of peritoneal recurrence [6]. The 3-year recurrence free survival for patients from each arm of the study was equivalent. The COLOPEC trial assessed the utility of prophylactic HIPEC in patients at high risk of PM [7]. Patients with advanced CRC or perforated tumor without PM were randomized to receive adjuvant HIPEC followed by systemic chemotherapy or adjuvant systemic chemotherapy alone. The 18-month PM-free survival was equivalent for both arms in this study.
University Hospitals Cleveland Medical Center is a tertiary care teaching hospital that developed a HIPEC program in 2011. Patient selection for this procedure has evolved over time due to the cumulative experience of surgical oncologists and the release of results from key clinical trials such as PRODIGE-7. We aimed to study our institutional experience with HIPEC focusing on the evolution in patient selection along with publication of these major HIPEC studies.
Methods
IRB Approval
Prior to data collection, approval was obtained from the institutional review board of the University Hospitals Cleveland Medical Center (STUDY20221444).
Data Source
We retrospectively reviewed cases of adult patients who underwent CRS with or without HIPEC by the surgical oncology team at the University Hospitals Cleveland Medical Center between 2011 and 2022. The demographic characteristics, tumor profile, pre-, intra-, and post-operative, and survival data were also recorded in the database. Comorbidities were documented using Charlson Comorbidity Index (CCI) [8]. We limited our analysis to cases performed by a single surgical oncologist as more than 90% of these operations were performed by a single surgeon providing the best representation of evolving practice patterns over time.
Patient Characteristics
We stratified the entire patient sample into two time periods based on time of CRS. The first group consisted of patients who underwent CRS before the results of PRODIGE-7 trial (2011-2018) whereas the second group consisted of those undergoing CRS after 2018 (2018-2022). The extent of peritoneal metastases was estimated using peritoneal carcinomatosis index (PCI) whereas completeness of cytoreduction score (CC score) was recorded to assess the extent of tumor eradication [9,10].
Outcome Measures
Our primary outcome of interest was the change in utilization of HIPEC. The secondary outcome was any post-operative adverse event within 90 days of CRS-HIPEC, defined as any post-operative morbidity, readmission, and mortality.
Statistical Analysis
Clinicodemographic characteristics were compared using descriptive statistics. Overall survival was defined as the time from the date of CRS-HIPEC to the date of last contact or death. Recurrence-Free-Survival (RFS) was defined as the time from date of CRS-HIPEC to the date of first recurrence or death, whichever was earlier. A multivariable logistic regression model was computed for factors associated with composite outcome of post-operative morbidity within 90 days. The multivariable model was adjusted for factors significantly associated with the composite outcome on univariable analysis (age, sex, CCI, before or after PRODIGE-7, HIPEC). Survival functions for each time were computed using Kaplan-Meier method and compared between those treated before and after PRODIGE-7 using log rank test.
Results
A total of 88 patients underwent CRS with or without HIPEC between 2011 and 2022. Of these, 82 were operated on by a single surgeon. 67 (77%) patients underwent HIPEC, most commonly using mitomycin C (n=57, 85%). Among all patients, PM were most commonly of appendiceal (n=47, 55%) and colorectal (n=25, 29%) origin. The clinicodemographic characteristics are summarized in Table 1.
Total Number of Patients
88
Age
n (%)
0-40
5 (6%)
41-50
20 (23%)
51-60
20 (23%)
61-70
32 (36%)
71-
11 (13%)
Sex
Male
45 (51%)
Female
43 (49%)
Primary Site
Appendiceal
47 (55%)
Colorectal
25 (29%)
Mesothelioma
10 (12%)
Small Bowel
2 (2%)
Gastric
1 (1%)
Unknown Primary Site
1 (1%)
Histology
Mucinous Adenocarcinoma
32 (38%)
Adenocarcinoma
27 (32%)
LAMN
10 (12%)
Epithelioid Mesothelioma
6 (7%)
Goblet Cell Adenocarcinoma
4 (5%)
Papillary Mesothelioma
3 (4%)
Signet ring cell adenocarcinoma
1 (1%)
Serous Carcinoma
1 (1%)
Prior Cytoreductive Surgery
No
84 (95%)
Yes
4 (5%)
Charlson Comorbidity Index
Median (IQR)
3 (2, 4)
Neoadjuvant Chemotherapy
No
43 (48.9%)
Yes
43 (48.9%)
Unknown
2 (2.3%)
Response to Neoadjuvant Chemotherapy
Stable
19 (48%)
Improved
14 (35%)
Progressed
7 (18%)
Time period of CRS
Before PRODIGE-7 (2011-2018)
50 (57%)
After PRODIGE-7 (2019-2022)
38 (43%)
PCI range
<10
40 (48%)
20-Oct
30 (36%)
>20
13 (16%)
PCI Median (IQR)
10 (4-18.5)
CC Score Median (IQR)
0 (0, 1)
HIPEC
67 (77%)
HIPEC Regimen
Mitomycin C
57 (85%)
Doxorubicin + Cisplatin
9 (13%)
Cisplatin
1 (1%)
Table 1: Clinicodemographic features of patients who underwent CRS between 2011 and 2022.
50 (57%) patients underwent CRS-HIPEC before PRODIGE-7 and 38 (43%) were treated after PRODIGE-7. The median PCI and CC scores for all patients were 10 (interquartile range: 4-18.5) and 0 (IQR: 0-1), respectively. The median PCI for patients who underwent CRS-HIPEC increased from 8 (IQR:3-17) to 13 (IQR: 3-19) after PRODIGE-7. There was a corresponding increase in median CC score from 0 (IQR: 0-0) to 1 (IQR:0-2) between these time periods.
Patient Selection for HIPEC
The characteristics of patients treated in the time period before and after PRODIGE-7 are summarized in Table 2. The proportion of patients undergoing HIPEC along with CRS decreased after PRODIGE-7 (n=44, 90% vs. n=18, 56%). There was corresponding reduction in the use of HIPEC among patients requiring major gastrointestinal (GI) visceral resections after PRODIGE-7. Among patients who underwent colectomy, HIPEC was only used in 6 (26%) compared to 25 (57%) among those treated before PRODIGE-7. The types of procedures that patients in our cohort underwent are summarized in the supplemental table (Table S1).
CRS alone
CRS+HIPEC
P-value
Total Number of Patients
5 (10%)
44 (90%)
Any morbidity/mortality within 90 days
5 (100%)
21 (48%)
0.026
Pulmonary
Pneumo/hemothorax
0 (0%)
1 (1%)
Renal
UTI
0 (0%)
1 (2%)
Renal Failure
0 (0%)
0 (1%)
Vascular
DVT/PE
1 (20%)
1 (2%)
Gastrointestinal
Abscess/Fistula/Leak
0 (0%)
4 (2%)
Ileus/Obstruction
1 (20%)
6 (24%)
Persistent Diarrhea
0 (0%)
0 (0%)
Infection
Wound Infection/Dehiscence
0 (0%)
3 (7%)
Blood loss
1 (20%)
1 (2%)
Postoperative Bleeding requiring invasive intervention
0 (0%)
0 (0%)
Other Complications
2 (40%)
5 (14%)
Reoperation
0 (0%)
3 (8%)
0.61
Readmission to ICU
1 (20%)
1 (3%)
0.049
Blood Transfusion
2 (40%)
9 (20%)
0.32
Readmission within 90 days
1 (20%)
7 (17%)
0.85
Mortality within 90 days
0 (0%)
1 (2%)
0.75
PCI score, median (IQR)
10 (6, 10)
8 (3, 17)
0.53
CC score, median (IQR)
0 (0,0)
0 (0,0)
0.62
Primary Site
Appendiceal
4 (80%)
23 (52%)
0.54
Colorectal
0 (0%)
15 (34%)
Gastric
0 (0%)
1 (2%)
Mesothelioma
1 (20%)
4 (9%)
Unknown
0 (0%)
1 (2%)
Histology
Adenocarcinoma
0 (0%)
16 (36%)
0.42
Mucinous Adenocarcinoma
4 (80%)
22 (50%)
Mesothelioma
1 (20%)
3 (7%)
LAMN
0 (0%)
1 (2%)
Other
0 (0%)
- (5%)
Table 2: Postoperative adverse events by time period. A Before PRODIGE-7 (2011-2018)
CRS alone
CRS+HIPEC
P-value
Total Number of Patients
14 (44%)
18 (56%)
Any morbidity/mortality within 90 days
8 (57%)
5 (28%)
0.039
Pulmonary
Pneumo/hemothorax
0 (0%)
1 (20%)
Renal
UTI
0 (0%)
0 (0%)
Renal Failure
0 (0%)
1 (20%)
Vascular
DVT/PE
1 (12.5%)
0 (0%)
Gastrointestinal
Abscess/Fistula/Leak
1 (12.5%)
0 (0%)
Ileus/Obstruction
2 (25%)
0 (0%)
Persistent Diarrhea
0 (0%)
0 (0%)
Infection
Wound Infection/Dehiscence
0 (0%)
1 (20%)
Blood loss
2 (25%)
1 (20%)
Postoperative Bleeding requiring invasive intervention
0 (0%)
1 (20%)
Other Complications
2 (25%)
0 (0%)
Reoperation
1 (8%)
1 (6%)
0.83
Readmission to ICU
2 (18%)
1 (6%)
0.33
Blood Transfusion
3 (21%)
5 (28%)
0.68
Readmission within 90 days
4 (29%)
2 (11%)
0.21
Mortality within 90 days
1 (7%)
0 (0%)
0.25
PCI score, median (IQR)
14.5 (6, 21)
13 (3, 19)
0.35
CC score, median (IQR)
1 (0, 2)
1 (0, 2)
0.84
Primary Site
Appendiceal
7 (50%)
10 (56%)
0.29
Colorectal
4 (29%)
5 (28%)
Mesothelioma
0 (0%)
3 (17%)
Small Bowel
1 (7%)
0 (0%)
Unknown
1 (7%)
0 (0%)
Histology
Adenocarcinoma
4 (29%)
4 (22%)
0.29
Mucinous Adenocarcinoma
4 (29%)
2 (11%)
Mesothelioma
0 (0%)
3 (17%)
LAMN
2 (14%)
6 (33%)
Other
1 (7%)
3 (17%)
Table 2 of 1: B.After PRODIGE-7 (2019-2022)
Post-operative Adverse Events
The most common postoperative complications within 90 days were ileus and obstruction (n=9, 10%) followed by formation of gastrointestinal abscess or fistula (n=5, 6%). Overall, 5 (7%) patients required reoperation as well as readmission to the intensive Care Unit (ICU). 2 (2%) of the patients died within 90 days of surgery. The post-operative outcomes are summarized in the supplemental table (Table S2).
On multivariable analysis, postoperative adverse events were more likely for patients with PCI of 20+ (OR:13.62, 95% CI=2.09-88.57, p=0.006) and less likely for patients treated after PROIDGE-7 (OR:0.28, 95% CI=0.08-0.98, p=0.047) and underwent HIPEC (OR:0.17, 95% CI=0.04-0.72, p=0.016) (Table 3).
Odds ratio
95% CI
P-value
Age
0-40
Reference
41-50
1.67
0.09
31.78
0.732
51-60
3.73
0.19
74.01
0.388
61-70
2.17
0.11
43.84
0.614
71-100
3.99
0.14
115.07
0.42
Sex
Male
Reference
Female
0.33
0.1
1.04
0.058
Charlson-Comorbidity Index
Per point
0.93
0.7
1.22
0.591
PCI Score
<10
Reference
10-20
1.85
0.58
5.87
0.297
>20
13.62
2.09
88.57
0.006
Time Period
Before PRODIGE-7
Reference
After PRODIGE-7
0.28
0.08
0.98
0.047
HIPEC
No
Reference
Yes
0.17
0.04
0.72
0.016
Table 3: Factors associated with any morbidity/mortality within 90 days of surgery.
Survival
As shown in Figure 1A, patients treated before and after PRODIGE-7 demonstrated equivalent RFS (median RFS: 14.3 vs. 18.5 months, p=0.357). The median OS for those treated before PRODIGE-7 was 86.6 months (Figure 1B). Patients treated after PRODIGE-7 did not reach a median for OS due to the short follow-up time.
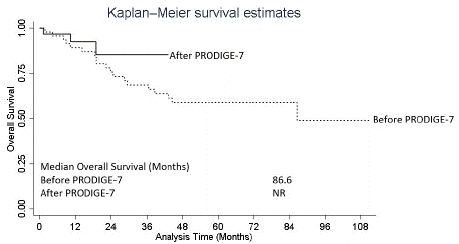
Figure 1A: Recurrence-Free Survival.
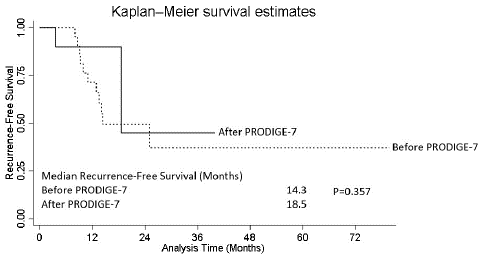
Figure 1B: Overall Survival.
Discussion
For some providers, the results of the key clinical trials such as PRODIGE-7 have called into question the role of HIPEC in treatment of PM. Patient selection has evolved over time since the development of a HIPEC program at our institution in 2011. In this study, we analyzed our institutional usage of HIPEC over time and found there was an overall reduction in HIPEC usage, most pronounced for patients with higher PCI and those requiring major GI visceral resection.
Recently published randomized trials (PRODIGE-7, PROPHYLOCHIP and COLOPEC) have demonstrated no survival benefit with the addition of HIPEC to CRS for patients with colorectal cancer. These trials were limited by their use of a 30-minute Oxaliplatin HIPEC regimen, inappropriate overall survival endpoints, and patient selection with respect to their disease burden [2]. Systemic Oxaliplatin is known to induce chemoresistance to HIPEC [11] and is associated with higher rate of major complications which may worsen the outcomes [12,13]. Moreover, 30 minutes is a suboptimal duration for microscopic tumor eradication given cytotoxicity of chemotherapeutic agents correlates with duration and temperature of exposure [14-16]. The results of these trials negatively influenced global practice pattern of CRS-HIPEC [17]. The number of patients undergoing HIPEC for PM declined in most countries and HIPEC was taken off national guidelines in some of them [17]. However, in a survey from 19 different countries, most experts still remained confident in the role of HIPEC for the treatment of colorectal PM despite the results of PRODIGE-7 [17].
The aforementioned trials caused a shift of expert opinion towards the use of Mitomycin C HIPEC regimens with a longer duration of infusion (60-100 minutes) [17]. Mitomycin C is known to have a favorable safety profile when compared to Oxaliplatin and shows a rapid rise in tissue concentrations in residual tumor deposits over prolonged periods [13,18]. The HIPECT4 trial aimed to address this debate by randomizing patients with locally advanced CRC (cT4N0-2M0) patients at high risk of PM to surgery and prophylactic HIPEC or surgery alone arms [19]. This design was similar to the COLOPEC trial, with HIPEC regimen being the most significant difference (Mitomycin C in HIPECT4, oxaliplatin in COLOPEC). HIPECT4 demonstrated statistically significant improvement in locoregional control with HIPEC compared to surgery alone (97.6% vs. 87.6%). It is worth mentioning that only 67.9% of patients had pT4 tumors on final exam, which explains the relatively low rate of locoregional recurrence. While this trial was designed to investigate the impact of prophylactic HIPEC on the development of PM, it begs the question about the efficacy of a similar approach among patients with known PM.
The Spanish GECOP-MMC is a multicenter trial currently enrolling patients to investigate the role of Mitomycin C HIPEC regimen in preventing peritoneal recurrence among patients with a limited disease burden [20].
With the use of recurrence-free survival and exclusion of patients not achieving complete cytoreduction, it is likely to correct the methodological flaws inherent to the design of PRODIGE-7. Additionally, the ongoing CAIRO 6 study is investigating the role of perioperative chemotherapy with HIPEC [21]. In the meantime, as results from these studies continue to develop, thoughtful decisions must be made regarding patient selection to optimize cancer outcomes and quality of life while minimizing morbidity and mortality.
Our study has several limitations. First, our study includes a relatively small sample size for patients undergoing CRS-HIPEC surgery due to the relative infrequency of the procedure. In addition to a small patient cohort, our study is limited to a single center which may not be reflective of surgical outcomes and practices at other institutions. Although we provided evidence for changes in patient selection pattern after PRODIGE-7, these conclusions may not be extrapolated to other hospitals since patient selection is at the discretion of the surgeon performing the CRS-HIPEC procedure. The controversial results of the PRODIGE-7 trial limits the generalizability of our study results since different practitioners may carry differing interpretations of PRODIGE-7 [22-24].
In conclusion, the role of HIPEC in treating patients with PM in conjunction with CRS remains inconclusive. After PRODIGE-7, there was a reduction in HIPEC usage at our institution for patients with higher PCI and those requiring major GI visceral resections.
Ongoing clinical trials will further clarify the role of HIPEC in the treatment of PM, and the impact of real-world practice patterns remains to be seen.
Author Statements
Conflict of Interest/Disclosure
The authors disclose no conflict.
Funding/Financial Support
The project did not receive any external funding.
References
- Desai JP, Moustarah F. Peritoneal Metastasis. In: StatPearls. StatPearls Publishing. 2022.
- Sommariva A, Tonello M, Coccolini F, De Manzoni G, Delrio P, Pizzolato E, et al. Colorectal Cancer with Peritoneal Metastases: The Impact of the Results of PROPHYLOCHIP, COLOPEC, and PRODIGE 7 Trials on Peritoneal Disease Management. Cancers. 2022; 15: 165.
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FAN, et al. Randomized Trial of Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy Versus Systemic Chemotherapy and Palliative Surgery in Patients With Peritoneal Carcinomatosis of Colorectal Cancer. J Clin Oncol. 2003; 21: 3737-3743.
- Dodson RM, McQuellon RP, Mogal HD, Duckworth KE, Russell GB, Votanopoulos KI, et al. Quality-of-Life Evaluation After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2016; 23: 772-783.
- Quénet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021; 22: 256-266.
- Goéré D, Glehen O, Quenet F, Guilloit JM, Bereder JM, Lorimier G, et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP–PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 2020; 21: 1147-1154.
- Klaver CEL, Wisselink DD, Punt CJA, Snaebjornsson P, Crezee J, Aalbers AGJ, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019; 4: 761-770.
- Charlson Comorbidity Index (CCI). MDCalc. 2022.
- Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999; 43: S15-25.
- Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon’s role. Langenbecks Arch Surg. 1999; 384: 576-587.
- Prabhu A, Brandl A, Wakama S, Sako S, Ishibashi H, Mizumoto A, Takao N, et al. Effect of oxaliplatin-based chemotherapy on chemosensitivity in patients with peritoneal metastasis from colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: proof-of-concept study. BJS Open. 2021; 5: zraa075.
- Zhang X, Wu Q, Wei M, Deng X, Gu C, Wang Z. Oxaliplatin versus mitomycin C in HIPEC for peritoneal metastasis from colorectal cancer: a systematic review and meta-analysis of comparative studies. Int J Colorectal Dis. 2020; 35: 1831-1839.
- Wisselink DD, Braakhuis LLF, Gallo G, van Grecenstein WMU, van Dieren S, Kok NFK, et al. Systematic review of published literature on oxaliplatin and mitomycin C as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer. Crit Rev Oncol Hematol. 2019; 142: 119-129.
- Levasseur LM, Slocum HK, Rustum YM, Greco WR. Modeling of the Time-Dependency of in Vitro Drug Cytotoxicity and Resistance1. Cancer Res. 1998; 58: 5749-5761.
- Facy O, Radais F, Ladoire S, Delroeux D, Tixier H, Ghiringhelli F, et al. Comparison of hyperthermia and adrenaline to enhance the intratumoral accumulation of cisplatin in a murin model of peritoneal carcinomatosis. J Exp Clin Cancer Res. 2011; 30: 4.
- Kirstein MN, Root SA, Moore MM, Wieman KM, Williams BW, Jacobson PA, et al. Exposure–response relationships for oxaliplatin-treated colon cancer cells. Anticancer Drugs. 2008; 19: 37.
- van de Vlasakker VCJ, Lurvink RJ, Cashin PH, Ceelen W, Deraco M, Goere D, et al. The impact of PRODIGE 7 on the current worldwide practice of CRS-HIPEC for colorectal peritoneal metastases: A web-based survey and 2021 statement by Peritoneal Surface Oncology Group International (PSOGI). Eur J Surg Oncol. 2021; 47: 2888-2892.
- Kuzuya T, Yamauchi M, Ito A, Hasegawa M, Hasegawa T, Nabeshima T. Pharmacokinetic characteristics of 5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J Pharm Pharmacol. 1994; 46: 685-689.
- Arjona-Sánchez A, Espinosa-Redondo E, Gutiérrez-Calvo A, Segura-Sampedro JJ, Perez-Viejo E, Concepcion-Martin V, Sanchez-Sampedro JJ, et al. Efficacy and Safety of Intraoperative Hyperthermic Intraperitoneal Chemotherapy for Locally Advanced Colon Cancer: A Phase 3 Randomized Clinical Trial. JAMA Surg. 2023; 158: 683-691.
- Pereira F, Serrano A, Manzanedo I, Perez-Viejo E, Gonzalez-Moreno S, Gonzalez-Bayon L, et al. GECOP-MMC: phase IV randomized clinical trial to evaluate the efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) with mytomicin-C after complete surgical cytoreduction in patients with colon cancer peritoneal metastases. BMC Cancer. 2022; 22: 536.
- Rovers KP, Bakkers C, Simkens GAAM, Burger JWA, Nienhujis SW, Creemers GJM, et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parralel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer. 2019; 19: 390.
- An SL, Cai J, Wang H, Li Y. Complete cytoreductive surgery is the key to improving survival of colorectal cancer patients with peritoneal metastases: comment on PROPHYLOCHIP and PRODIGE 7. Zhonghua Wei Chang Wai Ke Za Zhi Chin J Gastrointest Surg. 2021; 24: 220-224.
- Sugarbaker PH, Van der Speeten K. The PRODIGE 7 randomized trial has 4 design flaws and 4 pharmacologic flaws and cannot be used to discredit other HIPEC regimens. J Gastrointest Oncol. 2021; 12: S129-S130.
- Ströhlein MA, Heiss MM. Limitations of the PRODIGE 7 trial. Lancet Oncol. 2021; 22: e178.