Research Article
Austin J Aquac Mar Biol. 2014;1(1): 10.
Replacement of Menhaden Fish Meal Protein by Solvent Extracted Soybean Meal and Soy Protein Concentrate Supplemented with L-Methionine and L-Lysine in the Diet of Juvenile Red Porgy Pagrus Pagrus
Alam MS1*, Watanabe WO1, Myers AR1, Rezek TC1, Carroll PM1 and Seaton PJ2
1Center for Marine Science, University of North Carolina Wilmington, USA
2Departments of Chemistry and Biochemistry, University of North Carolina Wilmington, USA
*Corresponding author: Alam MS, University of North Carolina Wilmington, Center for Marine Science, Aquaculture Program, 601 South College Road, Wilmington, NC, 28403-5927, USA.
Received: October 06, 2014; Accepted: November 17, 2014; Published: November 19, 2014
Abstract
Eight experimental diets were prepared to replace menhaden Fish Meal Protein (FMP, 59% crude protein) by solvent extracted Soy Bean Meal Protein (SBP, 47.5% crude protein) and Soy Protein Concentrate (SPC, 66% crude protein) for juvenile red porgy Pagrus pagrus. Five isonitrogenous (48%) and isolipidic (12%) diets were prepared replacing 0, 15, 30, 45 and 60% of FMP by SBP. In addition, three diets were prepared replacing 30, 45 and 60% of FMP by SPC. The control diet contained 66% menhaden fish meal, 0% SBP and 0% SPC. Crystalline L-methionine and L-lysine were added to the diets to simulate the calculated values of methionine and lysine found in the control diet. Fifteen fish were stocked in each of twenty-four 75-L tanks, and each test diet was fed to triplicate groups of fish (mean weight = 2.21 ± 0.02 g) for 56 days. Fish were fed twice per day (09:00 and 16:00 h) to apparent satiation. Compared to the control diet, percent body weight gain, feed intake, feed conversion ratios and protein efficiency ratios were not significantly (P> 0.05) different for fish fed from 15-45% SBP and 30-60% SPC, but were lower in fish fed 60% SBP. Broken line regression analysis, however, indicated an optimal substitution level of 34.9% for SBP. Survival of fish after the feeding trial ranged from 84 to 91% among treatments, with no significant differences. Apparent digestibility coefficients of protein in fish fed 60% SBP diet was significantly lower than control diet, but not the 60% SPC diet. Total essential amino acid and fatty acid composition of diets were similar in all test diets. Results indicated that the optimum level of FMP replacement by SBP and SPC were 34.9% and 45%, respectively, with supplemental Methionine and lysine in the diet of juvenile red porgy.
Keywords: Red porgy; Pagrus pagrus; Fish meal replacement; Alternative proteins and fish feed
Abbreviations
FMP: Fish Meal Protein; SBP: Soy Bean Meal Protein; SPC: Soy Protein Concentrate; UNCW: University of North Carolina Wilmington; CMS: Center for Marine Science; NOAA: National Oceanic and Atmospheric Administration; BWG: Body Weight Gain; SGR: Specific Growth Rate; FI: Feed Intake; FCR: Feed Conversion Ratio; PER: Protein Efficiency Ratio; EPA: Eicosapentaenoic Acid; DHA: Docosahexaenoic Acid; ARA: Arachidonic Acid; SFA: Saturated Fatty Acids; MUFA: Mono Unsaturated Fatty Acids; PUFA: Poly Unsaturated Fatty Acids; ADC: Apparent Digestibility Coefficient; ANF: Anti Nutrition Factor; CCFHR: Center for Coastal Fisheries Habitat Research
Introduction
The red porgy Pagrus pagrus, also known as sea bream, silver snapper, or pink snapper, is a valuable marine finfish in the family Sparidae, inhabiting the Mediterranean Sea, the eastern Atlantic (from British Isles to Senegal) and the western Atlantic (from North Carolina to Mexico and from Venezuela to Argentina) [1]. Red porgy is an important component of the snapper-grouper complex in the coastal Atlantic off the SE US (particularly NC and SC), red porgy populations have declined severely [2,3]. Due to declining natural populations, high market value, and suitability for intensive culture in tanks and in offshore cages, red porgy are considered a promising species for farming in the Mediterranean and Atlantic coastal areas [1,4-7]. Significant progress has been made in understanding the environmental and feeding requirement for larval culture of Atlantic red porgy [8,9] and production of post-metamorphicjuvenilesat University of North Carolina Wilmington (UNCW) [8]. Until recently, little or no experimental work has been conducted to elucidate the nutritional requirements of red porgy, or to develop cost-effective, environmentally-friendly diets for this species. Research is underway at UNCW to investigate the substitution limits of underutilized and locally available ingredients as alternative protein sources to fish meal that will help formulate a cost-effective and environmentally-friendly diet for commercial aquaculture of the red porgy.
Alternate plant protein sources to fish meal can lower the cost of aquaculture diets, reduce the amount of wild fish used as protein, and potentially reduce the nutrient levels in effluent waste [10,11]. However, for most species, there is a limit to how much fish meal can be replaced by alternative plant protein sources without negatively affecting the growth and feed efficiency [12]. Several authors have investigated the tolerance of fresh and saltwater fish for alternative protein sources. The maximum replacement levels of alternative plant protein sources for fish meal varies greatly depending on species [13]. Among alternative protein sources, soybean meal appears to be the most appropriate because of its ample supply, moderate price and favorable essential amino acid profile [14,15]. In addition to good amino acid profile, soybean meal has high protein content, very low carbohydrate and fiber, high digestibility compared to other plant protein sources [12,16]. Presently, soybean meal is the most important protein source in feeds for aquaculture species and as a partial or complete replacement for fish meal. Soybean meal is used not only because of its high protein content but also due to its worldwide availability.
The amino acid profile of soy protein is generally superior to other plant proteins; although compared to menhaden meal protein, it is lower in lysine and Methionine [16]. Although soy protein has a relatively balanced amino acid profile for fish, it is low in some essential amino acids especially methionine and lysine [14]. Thus, more attention has been focused on the beneficial effect of essential amino acid supplementation in soy-based diets on growth performance for several fish species [17-19]. Soybean meal has produced varying results in diets for many marine fishes, including salmonids Oncorhynchus mykiss [20]; giltheadsea bream Sparus aurata [21]; Japanese yellow tail Seriola quinqueradiata [22]; Mediterranean yellow tail Seriola dumerili [10]; red snapper Lutjanus argentimaculatus [23]; Japanese flounder Paralichthys olivaceus [24]; red drum Sciaenops ocellatus [25]; cobia Rachycentron canadum [26]; Atlantic cod Gadus morhua [27]; black sea bass Centropristis striata [19]; southern flounder Paralichthys lethostigma [18]; rose snapper Lutjanus guttatus [28] and yellow tail kingfish Seriola lalandi [29].
Soybean meal contains several Antinutritional Factors (ANF) that may affect the digestion or absorption of nutrients [12]. Thus, a few other soy protein sources are used in replacement of fish meal (especially for diets with a high protein content) since appropriate processing can eliminate or deactivate several ANF [30]. Soy protein concentrate is produced through aqueous ethanol or methanol extraction of defatted soy flakes, which typically contains 65-70% crude protein [31]. This additional extraction removes or deactivates ANF soluble carbohydrates and fiber [14]. Further, the extraction by alcohol can eliminate bitter off-flavors [32]. Fish meal has been partly or totally been replaced by SPC without adverse effects on growth performance in Atlantic salmon Salmo salar [33]; rainbow trout Oncorhynchus mykiss [20,34]; turbot Scophthalmus maximus L. [35]; Senegale sole Solea senegalensis [36] and Atlantic cod Gadus morhua [37].
Despite the recent increase in red porgy aquaculture research in the Mediterranean and Asia [38], no published data was available on red porgy culture with diets replacing fish meal by soybean meal protein. The objectives of this study were to investigate the effects of replacement of menhaden fish meal protein (59% crude protein) by solvent extracted Soybean Meal Protein (SBP, 47.5% crude protein) and soy protein concentrate (SPC, 66% crude protein)supplemented with methionine and lysine on growth performance, feed utilization and body composition of juvenile red porgy.
Materials and Methods
Experimental animals
Red porgy juveniles were produced at the University Of North Carolina Wilmington (UNCW)-Aquaculture Facility (Wrightsville Beach) from eggs collected from natural spawning of captive brood stock held at the Center for Coastal Fisheries Habitat Research (CCFHR) (National Ocean Service, NOAA) Laboratory (Beaufort, NC). These fish were hatched and reared at UNCW Aquaculture Facility according to published protocols [8] (Morris et al. 2008). Early juveniles were raised in 2.61-m3 in recirculating tanks until the feeding trial was conducted. Fish were fed a commercially prepared diet containing 50% protein and 15% lipid (Skretting, Vancouver, Canada) until the study commenced.
Experimental system
The experimental system consisted of twenty-four 75-L rectangular tanks supported by a recirculating aquaculture system located in an indoor climate-controlled laboratory. The recirculating aquaculture system included a Kaldness moving bed biofilter (Anox Kaldness Inc, Providence, Rhode Islands), a bead filter (Aquaculture Systems Technologies, LLC, New Orleans, Louisiana) to remove solids, a protein skimmer for removal of small particulate and dissolved materials and an ultra-violet sterilizer for disinfection. Temperature was controlled using a heat pump, and each tank was supplied with diffused air supplemented with pure oxygen when necessary. Dissolved oxygen, temperature, salinity and pH were measured using a multi-parameter probe (YSI 556 MPS, GEO Scientific Ltd., Vancouver, British Columbia). Total ammonia and nitrate were measured weekly using a portable data logging spectrometer (HACH DR/2010 SPEC, Loveland, CO, USA).
Experimental diets
Eight experimental diets were formulated to replace menhaden fish meal protein (59% crude protein) by solvent extracted soybean meal (47.5% crude protein) and soy protein concentrates (66% crude protein). Five isonitrogenous (48%) and isolipidic (12%) test diets (Table 1) were prepared replacing 0, 15, 30, 45 and 60% of menhaden Fish Meal Protein (FMP, 59% crude protein) by solvent extracted Soybean Meal Protein (SBP, 47.5% crude protein). The control diet (Diet 1) contained 66% menhaden fish meal, and all other nutrients in the diets were added according to the recent nutrients requirement information for marine fish [39,19]. Since menhaden fish meal (59 % protein) has a higher protein content than soybean meal (47.5 % protein), this was accomplished by reducing menhaden fish meal from control diet (Diet 1) by 0, 9.9 19.8, 29.7 and 39.6% and adding soybean meal to levels of 0, 12.2, 24.3, 36.5, and 48.0 in the diets, respectively (Table 1, Diets 1 to 5). In addition, three isonitrogenous (48%) and isolipidic (12%) test diets (Table 1, Diets 6 to 8) were prepared replacing 30, 45 and 60% of FMP by soybean protein concentrate (SPC, 66% crude protein). This was accomplished by reducing menhaden fish meal from control diet (diet 1) by 19.8, 29.7 and 39.6% and adding SPC to levels of 18.0, 27 and 36% in the diets, respectively (Table 1, Diets 6 to 8). To maintain isolipidic levels and to avoid a deficiency of highly unsaturated fatty acid profiles in all diets, menhaden fish oil content was increased as the fish meal level decreased. Crystalline L-methionine and L-lysine were added to the diets to simulate the calculated values of methionine and lysine found in the control diet. Equal quantities of Kadai vitamin and mineral premix (Tomita Pharmaceutical Company, Kagoshima, Japan) for marine fish were used in the diets. Diets were prepared at UNCW-CMS using a feed mixer (Kitchen Aid Inc., St. Joseph, Michigan), meat grinder (Jacobi-Lewis Co. Wilmington, North Carolina) and a drying oven [18,19].
Diet No.
1
2
3
4
5
6
7
8
0%
15%
30%
45%
60%
30%
45%
60%
SBP
SBP
SBP
SPC
SPC
SPC
SPC
Soybean meal a
0
12.2
24.3
36.5
48
0
0
0
Soy protein conc. b
0
0
0
0
0
18
27
36
Menhaden meal c
66
56.1
46.2
36.3
26.4
46.2
36.3
26.4
Wheat starch
10
8
5.9
2
0
10
10
10
Wheat glutend
10
10
10
10
10
10
10
10
Menhaden fish oile
4
5
6
7
8
6
7
8
Soybean lecithinf
1
1
1
1
1
1
1
1
Vitamin premix g
2
2
2
2
2
2
2
2
Mineral premix g
2
2
2
2
2
2
2
2
Di-calcium phosphate
0.5
0.5
0.5
0.5
0.5
0.5
0.5
0.5
Methionine
0
0.5
0.6
0.7
0.8
0.6
0.7
0.8
Lysine
0
0.5
0.6
0.7
0.8
0.6
0.7
0.8
Cellulose
4.5
2.23
0.9
1.29
0.5
3.13
2.84
2.56
Total
100
100
100
100
100
100
100
100
Analyzed crude protein and lipid composition (%)
Crude protein
49.4
48.4
48.8
49.9
48.5
49
48.6
48.6
Digestible protein
43.3
43.7
43.9
44.6
40.7
43.9
43.6
42.6
Total lipid
14.7
13.9
14.3
14.1
13.8
13.9
13.3
13.7
Gross energyh
15
15
15
15
15
15
15
15
(Calculated kJ g-1 diet)
Table 1: Compositions of diets (g 100 g-1). Diets indicate % FMP replaced with SBP or SPC.
Feeding protocol
Fifteen fish were stocked in each of twenty-one 75-L tanks (360 fish total). Fish were acclimated to laboratory conditions and were fed the control diet (0% SBP) for one week, and then each test diet was fed to triplicate groups of fish (average weight = 2.21 ± 0.02 g) for 56 days. Fish were fed twice per day (9:00 and 16:00 h) to apparent satiation, and the amount of diet fed was recorded. Tanks were siphoned daily or as needed. A 10: 14 h light: dark photoperiod was maintained. Water quality was verified twice weekly. During the feeding period, water temperature ranged from 21.2 to 23.9 C and dissolved oxygen ranged from 6.07 to 7.41 mg/L. The ranges of other water quality parameters in the experimental tanks during the experimental periods were as follows: pH 7.69 to 8.3, salinity35.6 to 36.3 gL-1, ammonia 0.25 to 0.34 mgL-1 and nitrite 0.07 to 0.13 mgL-1. Fish from each tank were weighed in bulk every 4 weeks. After 56 days of feeding, five fish from each tank were sacrificed, freeze dried, and stored at -80 C for whole body proximate composition analysis.
Apparent digestibility coefficient (%) of protein
To determine the Apparent Digestibility Coefficient (ADC) of crude protein of fish, eight test diets were prepared by adding 0.5% chromic oxide to the diets as an inert marker. Each diet was reformulated to included 0.5% chromic oxide which was offset by a reduction of cellulose. All other ingredients and formulations were the same as the diets used in the growth study (Table 1). After 56 days of feeding, 15 fish from each treatment (5 from each triplicate tank) were stocked in one tank. Eight chromic oxide based diets were fed two times a day (08:00 and 16:00 h) to the respective tank, and feces were collected every morning at 08:00 h for 10 days. Before the second (15:00 h) feeding, all tanks were siphoned to remove any uneaten diets and feces. Uneaten diets (if any) were also removed from the tanks after 30 min. of feeding. Feces were stored at freezer at -32°C to analyze apparent digestibility of protein. Chromic oxide content was determined using a spectrophotometer (Thermo Fisher Scientific, Two Rivers, Wisconsin, USA) through a modified Furukawa & Tsukahara (1966) method [40].
The ADC of protein was calculated using the formula:
ADC of protein (%) = 100 X [1-(Fprotein/ DproteinX Dchromic / Fchromic)]
Where F is the percent of proteinor chromic oxide in the fecal matter and D is the percent of proteinor chromic oxide in the diet.
Proximate composition
Proximate composition of all experimental diets and body tissues for all three experiments were analyzed at UNCW-CMS. Crude protein was determined by the Kjeldahl method with a Labconco Kjeltec System (Rapid Digestor, Distilling Unit-Rapid Still II and Titration Unit, Labconco Corporation, Kansas city, MO, USA) using boric acid to trap ammonia. Crude lipid (Soxhlet byether extraction), ash (BARNSTAD Thermolyne Muffle Furnace, IA, USA) and moisture (Fisher Scientific Isotemp Oven, Pittsburgh, PA, USA) contents in the diets were analyzed by standard methods [41]. Moisture contents in whole bodies were determined by drying the fish in a freeze dryer (Labcanco Freeze Dryer, Kansas city, MO, USA).
0%
15%
30%
45%
60%
30%
45%
60% Red Sea
SBP
SBP
SBP
SBP
SPC
SPC
SPC Breama
Threonine1.51
1.7
1.43
1.49
1.82
1.48
1.46
1.56
0.86
Valine1.75
1.97
1.68
1.71
2.08
1.72
1.67
1.82
1.2
Methionine1.04
1.55
1.3
1.34
1.58
1.33
1.3
1.45
1.052
Iso-leucine1.44
1.64
1.42
1.49
1.86
1.46
1.45
1.62
1.06
Leucine2.80
3.16
2.71
2.85
3.49
2.79
2.77
3.07
2.02
Phenylalanine1.62
1.94
1.71
1.84
2.35
1.75
1.78
2.01
1.963
Histidine0.95
1.09
0.93
0.96
1.18
0.95
0.93
1.03
0.67
Lysine2.66
3.26
2.88
2.92
3.73
2.98
2.9
3.19
2.11
Arginine2.31
2.65
2.31
2.46
3.1
2.35
2.37
2.65
1.68
Hydroxyproline
0.590.58
0.36
0.33
0.31
0.41
0.33
0.22
Aspertic acid3.23
3.78
3.3
3.55
4.49
3.38
3.43
3.88
Serine1.55
1.81
1.55
1.72
2.17
1.61
1.7
1.91
Glutamic acid6.74
7.77
6.71
7.5
9.33
6.99
7.33
8.26
Proline2.36
2.52
2.18
2.25
2.84
2.27
2.23
2.39
Glycine2.57
2.67
2.11
2
2.27
2.13
1.98
1.96
Alanine2.18
2.33
1.87
1.84
2.11
1.91
1.81
1.86
Tyrosine1.22
1.42
1.26
1.37
1.69
1.3
1.28
1.42
Table 2: Analyzed amino acid composition (g 100 g-1 dry diet) of test diets. Values are means (N=2). Diet indicates percentage of FMP replaced with SBP and SPC.
Amino acid analysis of diets
Total amino acid composition of the diets was conducted by AAA Service Laboratory (Damascus, OR, USA). Nor-leucine was used as internal standard. After hydrolysis, samples were analyzed using post-column derivatization on Hitachi L8900 analyzers (Hitachi High Technologies America, Inc. USA).
Fatty acid analysis
Fatty acid compositions of the diets were determined by first extracting total lipids in chloroform: methanol [42]. One ml of a 0.001 g mL-1 solution of C19:0 fatty acid was added to each sample as an internal standard. Lipid fatty acids were converted to their methyl esters (FAMEs) for GC analysis by refluxing the concentrated lipid sample in 1.0 mL of 0.5 M NaOH/MeOH for 30 min., followed by addition of 1.5 mL of boron trifluoride-methanol (BF3) and refluxing for an additional 30 minutes. The FAMEs were extracted into hexane, concentrated and re-dissolved in 1 mL of chloroform. GC analysis was performed on a HP-6890 Gas Chromatograph using a 25 meter x 0.25 μm HP-5 capillary column with FID detection (Department of Chemistry and Biochemistry, UNCW). Helium was used as the carrier gas. The column temperature profile was: 195 °C, hold for 8 min, ramp to 270 at 15 °Cmin-1and hold at 270 °C for 2 min. FAME peaks were integrated using the HP Chemstation soft ware package and individual FAMEs were identified by comparison of retention times to standards: GLC-84 (Nu Chek Prep U.S.A) as well as individual standards of steariodonic, eicosapentaenoic and arachidonic acid methyl esters (Sigma-Aldrich, MO, U.S.A). FAMEs from all samples were quantified using their peak areas compared to the peak area of the C19:0 internal standards.
Statistical analysis
All data were subjected to statistical verification by using one-way Analysis Of Variance (ANOVA) (JMP, version 7.0, SAS Institute, Cary, North Carolina). Significant differences between means were evaluated by Tukey-Kramer tests [43]. Probabilities of P <0.05 were considered significant. Broken-line regression analysis [44] was also used to determine the optimum dietary fish meal protein replacement by SBP. Regression analysis was performed using the software package JMP, version 7.0.
Results
Growth performance
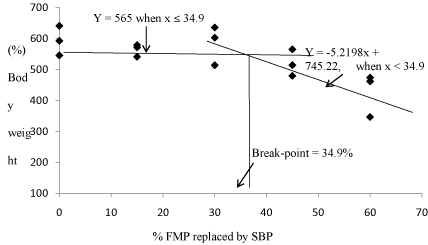
Table 4 shows the growth performance of fish fed the test diets after 56 days of the feeding trial. No significant differences (P< 0.05) were observed in Body Weight Gain (BWG) among the fish fed 0 to 45% SBP (520-593%) and 30 to 45% SPC (451-598%) diets (Table 4). However, compared to fish fed 0% SBP (593%), BWG decreased significantly for the fish fed 60% SBP (427%) and 60% SPC (451%). BWG for fish fed 60% SPC (451%) was also significantly lower than in fish fed 30-45% SPC (598-577%), but not significantly different from fish fed 45%SBP (520%). The optimum FMP replacement by SBP in the diet of juvenile red porgy based on BWG was found to be 34.9% by broken-line regression analysis (Figure 1).
Figure 1: Relationship between (%) body weight gain of red porgy juvenile and optimum % FMP replaced by SBP as described by the broken line regression model.The optimum value determined based on body weight gain for FMP replaced by SBP of juvenile red porgy was estimated to be 34.9%.
0%
15%
30%
45%
60%
30%
45%
60%
Fatty acids
SBP
SBP
SBP
SBP
SPC
SPC
SPC
14:00
5.23
5.05
5.26
4.68
4.65
4.69
4.76
4.88
16:00
15.55
14.88
15.4
12.5
12.38
13.15
13.4
12.98
16:1n-7
7.89
7.72
8.11
6.88
6.99
7.2
7.61
7.79
18:00
3.4
3.29
3.36
2.72
2.67
2.8
2.93
2.82
18:1n-9
7.44
7.72
8.45
7
7.22
6.94
7.5
7.59
18:1n-11
2.58
2.55
2.61
2.02
2.04
2.3
2.4
2.31
18:2n-6
5.93
6.84
8.31
7.6
8.27
5.56
6.07
6.22
18:4n-3
2.44
2.5
2.58
2.14
2.21
2.27
2.52
2.57
20:00
0.21
0.2
0.21
0.18
0.18
0.18
0.18
0.18
20:1n-9
0.92
0.95
0.99
0.82
0.84
0.88
0.96
0.97
20:2n-6
0.25
0.26
0.27
0.24
0.25
0.24
0.27
0.27
20:4n-6 (ARA)
0.8
0.71
0.67
0.45
0.44
0.62
0.68
0.62
20:5n-3 (EPA)
9.19
9.39
9.84
7.77
7.98
8.74
9.38
9.47
22:5n-3
1.83
1.88
2.04
1.46
1.5
1.71
1.87
2.1
22:6n-3 (DHA)
10.7
10.8
10.9
8.03
8.06
9.68
10.2
9.99
S SFA
24.4
23.4
24.2
20.1
19.9
20.8
21.3
20.9
S MUFA
18.8
18.9
20.2
16.7
17.1
17.3
18.5
18.7
S n-3 PUFA
24.2
24.6
25.3
19.4
19.8
22.4
23.9
24.1
S n-6 PUFA
6.98
7.81
9.25
8.29
8.96
6.42
7.02
7.11
n-3/n-6 PUFA
3.46
3.14
2.74
2.34
2.2
3.49
3.42
3.39
DHA/EPA
1.17
1.15
1.1
1.03
1.01
1.11
1.09
1.05
Table 3: Fatty acid composition of diets (mg g-1 dry diets).Values are means (N=2). Diet indicates percentage of FMP replaced with SBP and SPC.
Diets
(%) BWG
SGR
FI
FCR
PER
SR
0%
593 ±28a
2.47±0.02a
0.22±0.01ab
0.97 ±0.02bc
2.09 ±0.02ab
91±2.0
15%
SBP564 ±12a
2.41±0.02a
0.22±0.01ab
1.01 ±0.03abc
2.02±0.02abc
89±5.8
30%
SBP584 ±36a
2.41±0.04a
0.24±0.02a
1.03 ±0.03abc
1.97±0.05abc
87±3.7
45%
SBP520 ±25ab
2.31±0.05ab
0.21±0.01abc
1.01 ± 0.02abc
2.02±0.05abc
85±2.3
60%
SBP427 ±40c
2.08±0.11c
0.18±0.01c
1.16 ± 0.05a
1.86±0.11c
84±4.3
30%
SPC598 ±37a
2.42±0.07a
0.24±0.03a
1.01 ± 0.03abc
2.02±0.07abc
87±3.7
45%
SPC577 ±26a
2.42±0.05a
0.21±0.03abc
0.94 ± 0.04c
2.18± 0.05a
91±5.8
60%
SPC451 ±26bc
2.17±0.08bc
0.19±0.02bc
1.10 ± 0.03a
1.93±0.08bc
84±4.3
Table 4: Percent body weight gain, Specific Growth Rate (SGR), feed intake, Feed Conversion Ratio (FCR), percent survival of juvenile red porgy fed different diets for 56 days. Diet indicates percentage of FMP replaced with SBP and SPC. Values are means ± SEM (N=3). Means with different letters in the same column differ significantly (P< 0.05).
Survival of fish after the feeding trial was 84 to 91% with no significant differences among the groups (Table 4). Specific Growth Rate (SGR) followed a similar trend to BWG, with significantly lower values at the 60% FMP substitution level for both SBP (2.08% d-1) and SPC (2.17%d-1) than in the high fish meal control diet (2.47% d-1) and with no significant differences among SBP and SPC diets at the lower substitution levels (Table 4).
Feed intake(FI, g/fish/d)was significantly lower for fish fed the 60% SBP diet (0.18) than for fish fed 0 to 30% SPB diets (0.22-0.24). However, feed intake for the fish fed 15 to 45% SBP (0.21 - 0.24) and 30 to 45% SPC (0.21 - 0.24) diets were not significantly different from those fed the 0% control diet (0.22). The lowest Feed Conversion Ratio (FCR) (0.94) was found for fish fed the 45% SPC diet, but was not significantly different from fish fed 0 to 45% SBP (0.97 - 1.03) and the 30% SPC diet (1.01). However, FCR was significantly higher for the 60% SPC diet (1.10) and the 60% SBP diet (1.16) than in the 0% control diet (0.97). Protein Efficiency Ratio (PER) was significantly lower for the fish fed 60% SBP diet (1.86) than for fish fed the 0% diet (2.09). However, PERs among the fish fed 0 to 45% SBP (1.97 - 2.09) and 30-45% SPC (2.02-2.18) were not significantly different (Table 6).
Diet
Protein
Lipid
Ash
0%
61.5 ± 0.34
18.0 ± 0.21b
18.0 ± 0.11ab
15% SBP
62.9 ± 0.53
17.0 ± 0.39c
18.5 ± 0.22a
30% SBP
61.5 ± 0.96
18.8 ± 0.21a
18.6 ± 0.28a
45% SBP
61.5 ± 0.74
18.0 ± 0.23b
18.3 ± 0.18ab
60% SBP
60.8 ± 0.20
16.1 ± 0.05d
18.6 ± 0.14a
30% SPC
62.2 ± 0.36
18.7 ± 0.30ab
17.9 ± 0.03ab
45% SPC
61.3 ± 0.24
17.9 ± 0.27b
17.6 ± 0.13b
60% SPC
61.5 ± 0.16
17.2 ± 0.21c
17.6 ± 0.26b
Table 5: Effects of replacement of FMP (0-60%) by SBP and SPC on whole body proximate composition (% dry basis) of red porgy). Diet indicates percentage of FMP replaced with SBP and SPC. Values are means ± SEM (N = 3). Means with different letters in the same column differ significantly (P< 0.05).
Diets
ADC of Protein (%)
0%
87.7 ± 0.59b
15% SBP
90.3 ± 0.49a
30% SBP
90.0 ± 0.29a
45% SBP
89.4 ± 0.53a
60% SBP
83.9 ± 0.40c
30% SPC
89.5 ± 0.69a
45% SPC
89.7 ± 0.56a
60% SPC
87.8 ± 0.31b
Table 6: Apparent Digestibility Coefficient (%) (ADC) of protein in fish fed the test diets. Diet indicates percentage of FMP replaced with SBP and SPC. Values are means ± SEM (N = 3). Means with different letters in the same column differ significantly (P< 0.05).
Apparent digestibility coefficients (%) of protein
The Apparent Digestibility Coefficients (ADC) (%) of protein in fish fed 15, 30 and 45% SBP (89.4-90.3%) were significantly higher than in fish fed the 0% control and 60% SBP diets (83.9-87.7%) (Table 7). The ADC of protein in fish fed 30 and 45% SPC (89.5-89.7%) were also significantly higher than the control and 60% SPC (Table 7).
Whole body proximate composition
Whole body crude protein content was similar (61.3-62.9%) among fish in all diet treatments, with no significant differences (Table 5). Whole body lipid content was also similar among diet treatments, but was significantly lower in fish the fed 60% SPB diet (16.1%) than in fish fed the other test diets (17.2 - 18.8%). The whole body lipid in fish fed 45% SBP (18.0%) and 45% SPC (17.9%) were not significantly different from fish fed the 0% control diet (18.0%). Whole body ash content in fish fed15, 30 and 60% SBP diets were significantly higher than the fish fed 45 and 60% SPC diets (17.6%) (Table 5).
Amino acid composition of diets
Methionine and lysine level in all diets were ranged from 1.04 to 1.58%and2.66 to 3.73%of dry diet, respectively (Table 2). Ranges of other essential amino acids were: valine (1.67-2.08%), iso-leucine (1.42 - 1.64%), leucine (2.77 - 3.49%), phenyl alanine (1.62 - 2.35%), histidine (0.93 - 1.18%) and arginine (2.31 - 3.1%) (Table 2).
Fatty acid composition of diets
Total Saturated Fatty Acids (SFA) ranged from 19.9 - 24.4 mg g-1 diet among experimental diets (Table 3). The 45-60% SBP diets contained higher in linoleic acid (18:2n-6) (6.07 - 8.31 mg g-1) than the 0% SBP diet (5.93 mg g-1) and the 30% SPC diet (5.56 mg g1). The concentration of n-3 Poly Unsaturated Fatty Acids (PUFA) ranged from 19.4-25.3 mg g-1 among treatments and was slightly lower in 45 and 60% (19.4-19.8 mgg-1) SBP diets than in other diets (Table 3). Arachidonic acid (20:4n-6) (0.44-0.45), Eicosapentaenoic Acid (EPA, 20:5n-3) (7.77-7.98mg g-1) and Docosahexaenoic Acid (DHA, 22:6n- 3) (8.03-8.06mg g-1) concentrations for the 45 and 60% SBP diets were lower than in the 0% SBP diet (0.80, 9.19 and 10.7mg g-1, respectively). DHA/EPA ratios ranged from 1.01 - 1.15, with no big differences among diets (Table 3).
Discussion
No statistically significant differences were found in % body weight gain among fish fed the 0, 15, 30 and 45% SBP diets (Table 4), whereas body weight gain was significantly lower for fish fed a 60% SBP diet than in those fed the 0%SBP control diet. However, a broken line regression analysis of percent body weight gain showed a more conservative optimum replacement level of FMP by SBP of 34.9% (Figure 1). Varying levels of SBP have been incorporated successfully (i.e., without reduction of growth performance) into the diets of other marine and freshwater species in replacement of FMP. In the present study, the optimum replacement level of FMP with SBP (34.9%) in red porgy diets was lower than the values reported for other carnivorous marine finfish species, such as Japanese flounder (45%) [24], cobia (50%) [26], Atlantic cod (50%) [45]; herbivorous freshwater fish, such as carp Cyprinus carpio (100%) [46], and Nile tilapia Oreochromis niloticus (100%) [47]. The optimum replacement level of FMP with SBP for red porgy in the present study is higher than reported for higher than reported for the yellowtail (20%) [22].
A comparison of percent weight gain data by ANOVA suggested that the maximum replacement of FMP by SPC in the red porgy diet was not more than 45% (Table 4). This FMP replacement level (45%) by SPC is higher than the value reported for juvenile black sea bream (40%) [48], turbot (25%) [35], and Gilthead Sea bream (30%) [17].
No significant differences were found in feed intake, FCR and PER among the groups fed 0 to 45% SBP and SPC diets, but significantly lower feed intake was observed in the fish fed the 60% SBP and SPC diets (Table 4) compared to the other test diets, indicating reduced palatability of diets replacing 60%FMP by SBP or SPC. This is similar to what was reported in yellow perch fed diets replacing 63.5% of the FMP by SBP [49]. Lower feed intake of red porgy fed the 60% SBP and SPC diets is a primary reason for poor growth at high replacement levels. In addition, FCR for red porgy fed the 60% SBP and SPC diets was significantly higher than in the 0% SBP control diet. Gilthead sea bream fed diets with 60% or 75% FMP replaced by SBP also showed higher FCR compared to fish fed the 0% SBP control diet containing only FMP [50]. PER in the 60% SBP diet was significantly lower than the control diet (Table 4). In Mediterranean yellowtail [10] and gilthead sea bream [50], PER declined at 40 and 45% replacement of FMP by SBP, respectively. It has been suggested that the poor growth performance and low feed utilization of fish fed high substitution levels of soybean protein for fish meal could be due to lower digestibility of nitrogen and energy, the presence of non-digestible oligosaccharides, mineral deficiencies, amino acid deficiencies and anti-nutritional factors [14]. Results of the present study suggest that SBP in the 60% FMP replacement diets was not as well digested and assimilated by red porgy as in diets containing higher levels of protein originating from soybean meal. In Europeanseabass, replacement of FMP by soy protein at levels above 50% negatively affected feed intake, feed efficiency, specific growth rate and protein retention ratio [35]. Protein intake is directly proportional to the feed intake, which in turn affects growth rate [51]. Low feed intake driven by a poor feed palatability appeared to have been a major factor restricting higher inputs of SBP and SPC in diet formulation of snapper [52], similar to the findings in the present study for red porgy juveniles.
In this study, survival remained high (84-91%) throughout the study, with no significant differences. This is similar to what was reported for black sea bass [19], southern flounder [18], Atlantic cod [27] and rainbow trout [53], which showed no significant differences in survival when fed diets containing high levels of soybean meal. Kasper et al. [49] however, found a significant decline in the survival of yellow perch when fed diets with 92% and 100% of the FMP replaced by SBP.
The ability of red porgy to grow on diets with 45% substitution levels of SBP and SPC for FMP indicates a superior ability to digest SBP compared to other marine finfish such as yellowtail [22]. Juvenile red porgy fed 15-45% SBP and 30-45% SPC diets had significantly higher Apparent Digestibility Coefficient (ADC) of protein than red porgy fed control, 60% SBP and 60% SPC (Table 7). In general, the higher AADC for the15, 30 and 45% SBP and 30-45% SPC diets suggests that SBP and SPC with fish meal more digestible than only fish meal protein and thus well assimilated by red porgy. In several finfish species including red sea bream, gilthead sea bream and Japanese flounder it has been suggested that the high growth performance and high feed utilization of fish fed high substitution levels of soybean meal for fishmeal could be due to higher digestibility of nitrogen and energy [24,50]. On the other hand, the results of the present study showed that red porgy fed 60% SBP diets had significantly lower ADC of protein than the diets with lower SBP levels. Diets with high plant protein can reduce digestibility of nutrients in fish [54]. Reduced ADC of protein was reported for Atlantic cod when solvent-extracted soybean meal was included in diets [27,55]. Different soybean products, such as soy protein concentrate, extracted and toasted (defatted) soybean meal, full-fat soybean meals, or low oligosaccharides soybean meal, have produced different growth performance in fish because of the different anti-nutritional factors, amino acid availability, leaching of supplemented amino acids, anti-nutrient compounds, and/or poorly digestible carbohydrates and soluble fiber content present in these products [56,57]. In contrast to SBP red porgy fed the 60% SPC diet showed no significant differences in ADC from the 0%SPC control diets. Many studies showed that there were no effects of incorporation of SPC on protein digestibility [35,58].
Supplementation of lysine and methionine to compensate for the deficiency of essential amino acids is beneficial in improving amino acid balance and palatability in high soybean protein based diets [39,59,60]. Methionine and lysine level (g 100 g-1 dry diet) in all diets in the present study with red porgy were similar to or higher than the methionine and lysine requirement levels reported for the congeneric red sea bream Pagrus major (Table 2) [61]. All other essential amino acids were more or less similar to or higher than the requirement values reported for red sea bream [61]. Similar growth and feed intake among the 0, 15, 30 and 45% SBP and 0, 30 and 45% SPC diets indicated that supplemental methionine and lysine improved feed utilization, and growth performance to the level observed in fish fed the control diet that has only intact protein sources. Supplementation of methionine and lysine in soybean based diets improved growth performance of red sea bream, Pagrus major [62] and Japanese yellowtail [63]. The lysine and methionine requirements of red porgy are unknown at this time. In the present study, diets were formulated with supplemental methionine and lysine in diets to simulate the methionine and lysine level found in the control diet. The analyzed values for total amino acids of the different diets (Table 2) appear to have been adequate for good growth and survival of juvenile red sea bream [39,61,64,65]. The amino acid availability of soybean meal for red porgy was not investigated. However, lower amino acid availabilities in the 60% SBP and SPC diets could affect the digestion, absorption and metabolism of the nutrients as observed for other species [54,58,66]. Nevertheless, the amino acid bioavailability from crystalline or ingredient-bound essential amino acid sources could be different [67] and may help explain the poor growth results achieved in red porgy fed a high level (60%) of SBP and SPC. Some aquatic animals, such as carp Cyprinus carpio, channel catfish, Japanese prawn Penaeusjaponicus appear to utilize free amino acids less efficiently than protein-bound amino acids [26,68].
In the present study, whole body protein content was not affected by SBP or SPC replacement of FMP up to 60% (Table 5), similar to what was reported in yellow croaker Pseudosciaena crocea (Richardson) [15]; rainbow trout [53] and Indian major carprohu Lebeo rohita L [69]. Whole body lipid content, however, was significantly lower in fish fed the 60% SBP diets compared to the 0% SBP diet (Table 5). A decline in whole body lipid level may indicate increasing use of lipid for energy in fish fed diets high in SBP and SPC [56] and may be related to lower protein digestibility and utilization as a source of energy at high substitution levels of these soybean protein ingredients. However the apparent lipid digestibility coefficients of diets were not measured in this experiment which could have some relation between whole body lipid and lipid digestibility. Mangrove red snapper also had a lower whole body lipid level when 50% FMP was replaced by SBP in the diets [23] a level of SBP replacement that reduced growth in these fish.
Total n-3 PUFA levels (1.9 - 2.5%) of the diets were within the range (0.5% - 2.5%) typically required for juvenile marine fish [70]. As SBP and SPC contents were increased in the diets, fish oil was increased to compensate for the low lipid content of SBP and SPC, producing similar n-3 PUFA levels in all test diets (Table 3). Red drum requires 0.5% dry diet of n-3dietary PUFA [71]. Requirements for n-3HUFA in other marine species such as red sea bream, yellowtail Seriola lalandi and turbot range from 0.5 to 2.0% of dry diet [72,73].
In this study, the ratio of DHA to EPA in the diets (1.0-1.1) was within the range of 0.5 - 1.0 required by gilthead sea bream [70]. Although the DHA or EPA requirements for juvenile red porgy are not known, based on the requirements of other marine finfish listed above, it appears that sufficient DHA and EPA was provided in all diets. There is no information on replacement of fish oil by vegetable oil in red porgy diets. However, these values meet the requirement (1.0 and 0.5%of the diet for DHA and EPA, respectively) for juvenile red sea bream as estimated by Takeuchiet al [74]. While ARA requirements for red porgy are unknown, the ARA concentrations in the test diets used in the present study ranged from 0.44 to 0.80%dry weight, lower than what was reported to maximize survival in juvenile gilthead sea bream (1.8% dry weight) [70,75]. Additional studies are needed to determine maximum fish oil replacement by vegetable oil in high soybean based diets for red porgy.
In summary, results suggested that the maximum levels of FMP replacement with SBP and SPC in red porgy diets were34.9% and 45%, respectively, when menhaden fish meal was used as a sole protein source in the control diet.
Acknowledgment
This research was supported by the Saltonstall-Kennedy Grant Program (FY09), Grant Number: NA09NMF4270084 under National Marine Fisheries Service and National Oceanic and Atmospheric Administration, Department of Commerce, USA and MARBIONC (Marine Biotechnology in North Carolina) Center for Marine Science, University of North Carolina Wilmington, USA. The authors also thanks to Solae LLC, St. Louis, MO, USA for the donation of soy protein concentrate for this experiment.
References
- Mihelakakis A, Yoshimatsu T, Tsolkas C. Spawning in captivity and early life history of cultured red porgy, Pagrus pagrus. Aquaculture. 2001; 199: 333-352.
- SAFMC (South Atlantic Fishery Management Council). Stock assessment of red porgy off the southeastern United States. South East Data, Assessment, and Review (SEDAR). Report of Assessment Workshop. Beaufort, North Carolina. 2006.
- Vaughan DS, Prager MH. Severe decline in abundance of the red porgy (Pagrus pagrus) population off the southeastern United States. Fishery Bulletin. 2002; 100: 351-375.
- Kolios P, Kiritsis S, Katribusas N. Larval-rearing and growout of the red porgy (Pagrus pagrus) in the Riopesca hatchery (Greece). Hydrobiol. 1997; 358:321-325.
- Hernandez-Cruz CM, Salhi M, Bessonart M, Izquierdo MS, Gonzalez MM, Fernandez-Palacios H. Rearing techniques for red porgy (Pagrus pagrus) during larval development. Aquaculture.1999; 179: 489-497.
- Papandroulakis N, Kentouri M, Divanch P. Biological performance of red porgy (Pagrus pagrus) larvae under intensive rearing conditions with the use of an automated feeding system. Aquacult Int.2004; 12: 191-203.
- Roo FJ, Hernández-Cruz CM, Socorro JA, Fernández-Palacios H, Izquierdo MS. Advances in rearing techniques of Pagrus pagrus, (Linnaeus, 1758): comparison between intensive and semi-intensive larval rearing systems. Aquaculture Res. 2010; 41: 433-449.
- Morris JA, Rezek TC, McNeill NA, Watanabe WO. Aquaculture of the Atlantic Red Porgy. North American J of Aquacult. 2008; 70:184-191.
- Ostrowski A, Watanabe WO, Montgomery F, Rezek T, Shafer T, Morris J. Effects of salinity and temperature on the growth, survival, whole body osmolality, and expression of Na+/K+ ATPase mRNA in red porgy (Pagrus pagrus) larvae. Aquaculture. 2011; 314: 193-201.
- Tomas A, Gandara FDL, Garcia-Gomez A, Perez L, Jover M. Utilization of soybean meal as an alternative protein source in the Mediterranean yellowtail, Seriola dumerili. Aquacult Nutr. 2005; 11: 333-340.
- Trushenski JT, Kasper CS, Kohler CC. Challenges and Opportunities in Finfish Nutrition.North American J of Aquacult. 2006; 68: 122-140.
- Gatlin DM III, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, et al. Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquacult Res. 2007; 38: 551-579.
- Loachmann R, Kumaran S. Effect of practical diets with animal or vegetable protein sources and poultry oil or menhaden fish oil on adult fathead minnow in tanks. North American J of Aquacult. 2006; 68: 281-286.
- Storebakken T, Refstie S, Ruyter B. Soy products as fat and protein sources in fish feeds for intensive aquaculture. In: Soy in Animal Nutrition (ed. by J.K. Drackley), Federated Animal Science Society, Savoy, IL, USA. 2000. pp. 127-170.
- Ai Q, Mai K, Tan B, Xu W, Duan Q, Ma H, et al. Replacement of fish meal by meat and bone meal in diets for large yellow croaker, Pseudosciaena crocea. Aquaculture. 2006; 260: 255-263.
- Hardy RW. Worldwide fish meal production outlook and the use of alternative protein meals for aquaculture. In: VIII International Symposium on Aquaculture Nutrition, Nov 15- 17, Universidad Autonoma de Leon, Monterry, Leon, Mexico. 2006.
- Kissil GW, Lupatsch I, Higgs DA, Hardy RW. Dietary substitution of soy and rapeseed protein concentrates for fish meal,and their effects on growth and nutrient utilization in giltheadseabream Sparus aurata L. AquacultRes. 2000; 31: 595-601.
- Alam MS, Watanabe WO, Myers AR, Rezek TC, Carroll PM, Longfellow S. Effects of replacement of menhaden fish meal protein by solvent extracted soybean meal protein supplemented with or without L-methionine and L-lysine on growth performance and body composition of juvenile southern flounder. North American J of Aquacult. 2011; 73: 350-359.
- Alam MS, Watanabe WO, Sullivan KB, Rezek TC, Seaton PJ. Replacement of menhaden fish meal protein by solvent extracted soybean meal protein in the diet of juvenile black sea bass Centropristis striata supplemented with or without squid meal, krill meal, methionine and lysine. North American J Aquacult. 2012; 74: 251-265.
- Kaushik SJ, Cravedi JP, Lall SP, Sumpte J, Fauconnea B, Larochem M. Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture. 1995; 133: 257-274.
- Nengas I, Alexis MN, Davies SJ. High inclusion levels of poultry meals and related byproducts in diets for gilthead sea bream, Sparus aurata L. Aquaculture. 1999; 179:13-23.
- Shimeno S, Kumon M, Ando H, Ukawa M. The growth performance and body composition of young yellowtail fed with diets containing defatted soybean meal for a long period. BullJapan Soc Sci Fish.1993; 59: 821-825.
- Catacutan M, Pagador G. Partial replacement of fishmeal by defatted soybean meal in formulated diets for the mangrove red snapper, Lutjanus argentimaculatus. Aquacult Res. 2004; 35: 299-306.
- Kikuchi K. Use of defatted soybean meal as a substitute for fish meal in diets of Japanese flounder, Paralichthys olivaceus. Aquaculture.1999; 179: 3-11.
- Mc Googan BB, Gatlin III DM. Effects of replacing fish meal with soybean meal in diets for red drum Sciaenops ocellatus and potential for palatability enhancement. J World Aquacult Soc.1997; 28: 374- 385.
- Zhou QC, Mai KS, Tan BP, Liu YJ. Partial replacement of fishmeal bysoybean meal in diets for juvenile cobia (Rachycentron canadum). AquacultNutr. 2005; 11,175-182.
- Hansen AC, Karlsen O, Rosenlund M, Rimbach M, Hemre GI. Dietary plant protein utilization in Atlantic cod, Gadus morhua L. Aquacult Nutr. 2007; 13; 200-215.
- Silva-Carrillo Y, Hernández C, Hardy RW, González-Rodríguez B, Castillo-Vargasmachuca S. The effect of substituting fish meal with soybean meal on growth, feed efficiency, body composition and blood chemistry in juvenile spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Aquaculture. 2012; 364-365:180-185.
- Bowyer JN, Qin JG, Smullen RP, Adams LR, Thomson MJS, Stone DAJ. The use of a soy product in juvenile yellowtail kingfish (Seriola lalandi) feeds at different water temperatures: 1. Solvent extracted soybean meal. Aquaculture. 2013; 384-387: 35-45.
- Anderson RL, Wolf WJ. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J Nutr. 1995; 125: 581-588.
- Lusas EW, Riaz MN. Soy protein products: processing and use. J Nutr. 1995; 125: 573-580.
- Morr CV, Ha EYW. Processing to prevent formation of off flavoursin soy products. Comments Agric. Food Chem. 1991; 2: 247-260.
- Olli JJ, Krogdahl A, Vabenø A. Dehulled solvent-extractedsoybean meal as a protein source in diets for Atlantic salmon, Salmosalar L. Aquacult Res. 1994; 26: 167-174.
- Collins SA, Desai AR, Mansfield GS, Hill JE, Van Kessel AJ, Drew MD. The effect of increasing inclusion rates of soybean, pea and canola meals and their protein concentrates on the growth of rainbow trout: Concepts in diet formulation and experimental design for ingredient evaluation. Aquaculture. 2012; 344-349: 90-99.
- Day OJ, González HGP. Soybean protein concentrate as aprotein source for turbot Scophthalmus maximus L. Aquacult Nutri. 2000; 6:221-228.
- Aragao C, Conceicao LEC, Dias J, Marques AC, Gomes E, Dinis MT. Soy protein concentrate as a protein source for Senegalese sole (Solea senegalensis Kaup 1858) diets: effects on growth and amino acid metabolism of postlarvae. Aquacult Res. 2003; 34: 1443-1452.
- Refstie S, Førde-Skjærvik O, Rosenlund G, Rørvik KA. Feed intake, growth, and utilization of macromutrients and amino acids by 1- and 2-year old Atlantic cod (Gadus morhua) fed standard or bioprocessed soybean meal. Aquaculture 2006; 255:279-291.
- Roo FR, Hernandez-Cruz CR, Socorro JA, Fernandez- Palacios H, Izquierdo MS. Advances in rearing techniques of Pagrus pagrus,(Linnaeus, 1758): comparison between intensive and semi-intensive larval rearing systems. Aquacult Res., 2010; 41: 433-449.
- Kader MA, Koshio S, Ishikawa M, Yokoyama S, Bulbul M. Supplemental effects of some crude ingredients in improving nutritive values of low fishmeal diets for red sea bream, Pagrus major. Aquaculture. 2010; 308: 136-144.
- Furukawa A, Tsukahara H. On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility of fish feed. Bull Japan Soc Sci Fish. 1966; 32:502-506.
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 17th edi. Association of Official Analytical Chemists, Arlington, Virginia, USA, 2000.
- Folch J, Lees M, Sloane-Stanley GH. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J Biol Chem. 1957; 226: 497-509.
- Kramer CY. Extension of multiple range tests to group means with unequal number of replications.Biometrics. 1956; 12: 307-310.
- Zeitoun IH, Ullrey DE, Magee WT. Quantifying nutrient requirement of fish. J Fish Res Board Canada.1976; 33, 167-172.
- Walker AB, Sidor IF, O'Keefe T, Cremer M, Berlinsky DL. Partial replacement of fish meal with soy protein concentrate in diets of Atlantic cod. North American J Aquacul. 2010; 72: 343-353.
- Viola S, Mokady U, Rappapor U, Arieli Y. Partial and complete replacement of fish meal by soybean meal in feeds for intensive culture of carp. Aquaculture. 1982; 26: 223- 236.
- Deyab M, El-Saidy EL, Magdy M, Gaber A. Complete replacement of fish meal by soybean meal with dietary L-lysine supplementation for Nile tilapia Oreochromis niloticus (L.) fingerlings. J World AquacultSoc. 2002; 33: 297-306.
- Ngandzali BO, Zhou F, Xiong W, Shao QJ, Xu JZ. Effect of dietary replacement of fish meal by soybean protein concentrate on growth performance and phosphorous discharging of juvenile black sea bream Acanthopagrus schlegelis. AquacultNutr. 2011; 17: 526-535.
- Kasper CS, Watkins BA, Brown PB. Evaluation of two soybean meals fed to yellow perch (Perca flavescens). AquacultNutr. 2007; 13: 431-438.
- Martinez-llorens S, Vidal AT, Garcia IJ, Torres MP, Cerda MJ. Optimum dietary soybean level for maximizing growth and nutrient utilization of on-growing gilthead sea bream (Sparus aurata). Aquacult Nutr. 2008; 15: 320-328.
- Phumee P, Wei WY, Ramachandran S, Hashim R. Evaluation of soybean meal in the formulated diets for juvenile Pangasianodon hypophthalmus (Sauvage, 1878). Aquacult Nutr. 2011; 17: 214-222.
- Freitas LEL, Nunes AJP, Carmo AV. Growth and feeding responses of the mutton snapper,Lutjanus analis (Cuvier 1828), fed on diets withsoy protein concentrate in replacement of Anchovy fish meal. Aquacult Res. 2011; 42: 866-877.
- Bureau DP, Harris AM, Cho CY. The effects ofpurified alcohol extracts from soy products on feed intake and growth of chinook salmon, Oncorhynchus tshawytscha and rainbow trout, Oncorhynchus mykiss. Aquaculture. 1998; 161: 27-43.
- Francis G, Makkar HPS, Becker K. Antinutritional factors present in plantderivedalternate fish feed ingredients and their effects in fish. Aquaculture 2001; 199: 197-227.
- Refstie S, Helland SJ, Storebakken T. Adaptation to soybean meal in the diets for rainbow trout, Oncorhynchus mykiss. Aquaculture 1997; 153: 263-272.
- Krogdahl A, Bakke-McKellep AM, Baeverfjord G. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon, Salmo salar. Aquacult Nutr. 2003; 9: 361-371.
- Rawles SD, Gatlin DM III. Nutrient Digestibility of Common Feedstuffs in Extruded Diets for Sunshine Bass Morone chrysops♀☓M. saxatilis ♂. J World Aquacult Soc. 2000; 31: 570 - 579.
- Refstie S, Storebakken T, Baeverfjord G, Roem AJ. Long-term protein and lipid growth of Atlantic salmon (Salmo salar) fed diets with partial replacement of fish meal by soy protein products at medium or high lipid level. Aquaculture. 2001; 193: 91-106.
- Alam MS, Teshima S, Yaniharto D, Sumule O, Ishikawa M, Koshio S. Assessment of reference amino acid pattern for diet of juvenile red sea bream, Pagrus major. Aquacult Int. 2005; 13: 369-379.
- Chatzifotis S, Polemitou I, Divanach P, Antonopoulou E. Effect of dietary taurine supplementation on growth performance and bile salt activated lipase activity of common dentex, Dentex dentex, fed a FM/soy protein concentrate-based diet. Aquaculture. 2008; 275: 201-208.
- Forster I, Ogata HY. Lysine requirement of juvenile Japanese flounder Paralichthys olivaceus and juvenile red sea beam Pagrus major. Aquaculture. 1998; 161: 131-142.
- Takagi S, Shimeno S, Hosokawa H, Ukawa M. Effect of lysine and methionine supplementation to a soy protein concentrate diet for red sea bream, Pagrus major. Fish Sci. 2001; 67: 1088- 1096.
- Watanabe T, Aoki H, Watanabe K, Maita M, Yamagata Y, Satoh S. Quality evaluation of different types of non-fish meal diets for yellowtail. Fish Sci. 2001; 67: 461-469.
- Kader MA, Bulbul M, Koshio S, Ishikawa M, Yokoyama S, Nguyen BT, et al. Effect of complete replacement of fishmeal by dehulled soybean meal with crude attractants supplementation in diets for red sea bream, Pagrus major. Aquaculture. 2012. 350-353, 109-116.
- Uyan O, Koshio S, Teshima S, Ishikawa M, Michael FR, Ren T, et al. Effects of tuna muscle powder in diet on the growth and phosphorus loading of juvenile red sea bream, Pagrus major. Aquacult Sci. 2007; 55: 29-40.
- Rawles SD, Gaylord TG, McEntire ME, Freeman DW. Evaluation of poultry by-product meal in commercial diets for hybrid striped bass, Morone chrysops x Morone saxatilis, in pond production. J World Aquacult Soc. 2009; 40: 141-156.
- Paripatananont T, Boonyaratpalin M, Pengseng P, ChotipuntuP. Substitution of soy protein concentrate forfishmeal in diets of tiger shrimp Penaeus monodon. AquacultRes. 2001; 32: 369-374.
- Teshima S, Ishikawa M, Shah Alam M, Koshio S, Michael FR. Supplemental effects and metabolic fate of crystalline arginine in juvenile shrimp Marsupenaeus japonicus. Comp Biochem Physiol B Biochem Mol Biol. 2004; 137: 209-217.
- Khan MA, Jafri AK, Chadha NK, Usmani N. Growth and body composition of rohu (Labeo rohita) fed diets containing oilseed meals: partial or total replacement of fish meal with soybean meal. Aquacult Nutr. 2003; 9: 391-396.
- SargentJR, Tocher DR, Bell JG. The Lipids. In: Fish Nutrition (Halver, J.E. & Hardy, R.W. eds) 2002; 181-257. Elsevier Science, San Diego, CA.
- Lochmann RT, Gatlin DM. Essential fatty acid requirement of juvenile red drum (Sciaenops ocellatus). Fish Physiol Biochem. 1993; 12: 221-235.
- National Research Council (NRC). Nutrient Requirements of Fish. National Academy Press, Washington, DC, USA. 1993; 115.
- Lee SM, Lee JH, Kim KD. Effect of dietary essential fatty acids on growth, body composition and blood chemistry of juvenile starry flounder (Paralatichthys stellatus). Aquaculture. 2003; 225: 269-281.
- Takeuchi T, Toyota M, Satoh S, Watanabe T. Requirement of juvenile red sea bream Pagrus major for eicosapentaenoic and docosahexaenoic acids. Nippon Suisan Gakkaish. 1990; 56:1263-1269.
- Bessonart M, Izquierdo MS, Salhi M, Hernandez-Cruz CM, Gonzalez MM, Fernandez-Palacios H. Effect of dietary arachidonic acid levels on growth and survival of gilthead seabream Sparus aurata L. larvae. Aquaculture.1999; 179: 265-275.
- Blaxter K. Energy metabolism in animals and man. Cambridge University Press, Cambridge. 1989.
Citation: Alam MS, Watanabe WO, Myers AR, Rezek TC, Carroll PM, et al. Replacement of Menhaden Fish Meal Protein by Solvent Extracted Soybean Meal and Soy Protein Concentrate Supplemented with L-Methionine and L-Lysine in the Diet of Juvenile Red Porgy Pagrus Pagrus. Austin J Aquac Mar Biol. 2014;1(1): 10.