Abstract
Rheumatoid Arthritis (RA) is a chronic, inflammatory autoimmune disease causing synovial proliferation and joint destruction. Hesperidin, a citrus flavanoid has been reported to be effective on reduction of Tumor Necrosis Factor-alpha, key inflammatory mediator in RA. Hesperidin Solid Lipid Nanoparticle (SLN) were prepared and optimised by 32 factorial design using emulsification- probe sonication method. The optimised SLN were found to be spherical in shape with particle size of 279nm, zeta potential of 17.86mV and 29.60% drug content. A comparative study in arthritis induced rats using Complete Freund’s adjuvant (CFA) was performed. The outcome of radiography, histopathology and ELISA study were employed to evaluate the effect of formulation on male wistar rats. Hence topical and oral SLN formulation was successful in treatment of rheumatoid arthritis. But the comparative results of both the formulations so obtained did not differ significantly.
Keywords: Hesperidin; Rheumatoid arthritis; Solid lipid nanoparticle
Abbreviations
RA: Rheumatoid Arthritis; SLN: Solid Lipid Nanoparticle; CFA: Complete Freund’s Adjuvant; TNF- α: Tumor Necrosis Factor- Alpha
Introduction
Rheumatoid arthritis is a chronic, progressive inflammatory autoimmune disease causing synovial inflammation and joint destruction [1]. Pain, fever, malaise, stiffness and swelling of joints constitute the primary symptoms of RA. Synovitis is caused by the release of pro-inflammatory cytokines like TNF-α, IL-6 and IL-1 which further leads to cartilage destruction, bone erosion and finally joint deformities. TNF-α being a key inflammatory mediator triggers an increase in synovial proliferation and also production of other secondary mediators. Overproduction of TNF-α in affected joints causes an increase in synoviocyte proliferation and a cascade of secondary mediators involved in the recruitment of inflammatory cells which leads to joint destruction [2]. TNF-α also induces osteoclast differentiation. Natural flavanoids like Tridax Procumbens Flavonoid (TPFs) has been reported to reduce differentiation of osteoclast [3]. Although the specific cause of RA is unknown up till now but it is reported that patients suffering from this disease share a common amino acid sequence in an antigen–presenting region of Major Histocompatability Complex (MHC) class II human leukocyte antigen molecules are more susceptible to RA. The interference of this complexes results in activation and proliferation of T-cells or Tlymphocytes. Major role of T-cell in RA is stimulation of synovial cells (macrophage like synoviocytes, dendritic cells, fibroblastic synoviocytes and B lymphocytes) and also release of pro-inflammatory cytokines causing destruction of bones and cartilage [4]. IL-2 being one of the pro-inflammatory cytokines regulates the activation of T cells [5]. Steroids, NSAID’s, immunosuppressant’s, DMARD etc. are the mainstay treatment strategies for RA but have their own share of side effects and toxicities which has lead to consideration of powerful natural agents as alternative treatment strategy [6].
Natural products play a key role in discovery of several therapeutic substances. According to estimate by World Health Organisation, 80% of world’s population relies on traditional medicine [7].
Hesperidin is a bioflavonoid obtained from orange peel Citrus sinesis family Rutaceae. The flavanone glycoside hesperidin is composed of hesperetin (flavanone) and rutinose (disaccharide). Hesperidin deficiency in body may cause capillary leakiness, night time leg cramps. The pharmacological property of hesperidin includes anti inflammatory, antioxidant, hypolipidaemic, and anti carcinogenic and is also used in the treatment of varicose vein [8]. Hesperidin also possesses anti- rheumatic action. At dose of 160mg/ kg hesperidin causes amelioration of joint destruction, synovial hyperplasia and infiltration of inflammatory cells. In adjuvant arthritis rat (CFA induced rats) hesperidin inhibited the production of IL- 1, IL-6, and TNF-α and restored the suppression of T- lymphocyte proliferation and production of IL-2 at the same time [6]. Hesperidin also exhibits significant anti-inflammatory activity by modulating the prostaglandin synthesis and COX-2 gene expression pathways [9].
The uptake of hesperidin flavonoid both orally and topically is not possible as it possess very limited water solubility, poor bioavailability and also poor permeability. Nanocarrier’s especially solid lipid nanoparticles could be helpful to enhance bioavailability and bioefficacy of this flavonoid [10]. In RA lymphatic uptake of drug plays a major role in the treatment of this disease [11]. SLN being nanosized and lipidic in nature will be one of the best candidate for the lymphatic uptake of the drug to the inflamed synovium of the arthritic joint. Complete Freund’s Adjuvant (CFA) model is composed of inactivated and dried mycobacteria when injected subcutaneously or intraperitoneally stimulate production of TNF-α which results in infiltration of the synovial membrane [12].
SLN are the submicron colloidal carrier composed of a solid lipid core in which lipophilic drug is encapsulated. They are biocompatible and biodegradable in nature and bear potential to carry both lipophilic and hydrophilic drug. It has superiority over other colloidal nanocarrier in terms of control over release kinetics of drug, enhanced drug stability and bioavailability of entrapped drug [13]. Present work is an attempt at formulation optimization of hesperidin solid lipid nanoparticles and in-vivo evaluation of its activity upon oral as well as topical administration.
Materials and Methods
Materials
Hesperidin (≥80%) and Complete Freund’s Adjuvant (CFA) were purchased from Sigma-Aldrich. Rat TNF -α ELISA kit was purchased from Ray Biotech. All other excipients used were of analytical standards.
Methods
Preparation of hesperidin SLN dispersion: Hesperidin loaded Solid Lipid Nanoparticles (SLN) dispersion was prepared using emulsification – probe sonication method [14]. The lipid (stearic acid) and mixture of hesperidin and dimethyl formamide (DMF) were heated to 10°C above the melting point of lipid (70°C). The aqueous phase was prepared by dissolving tween 80 and distilled water and heated to the same temperature of the oil phase. Both the phases were mixed together and stirred at 500rpm using a mechanical stirrer for 15 minutes maintaining the above mentioned temperature. The resultant dispersion were subjected for probe sonication (Sonics vibra 750) for 30 minutes which was further evaluated for particle size, zeta potential, drug content etc. The concentration of lipid and surfactant were finalised using design expert 9.1 software. The composition of SLN dispersion is shown in (Table 1).
Ingredients
Concentration (%)
Hesperidin (≥80%)
1
Stearic acid
5-15
Tween 80
2-6
Distilled water
qs to 100 ml
Table 1: The composition of SLN dispersion.
Drug excipients interaction: The interaction between drug and excipients were evaluated using Fourier Transform Infrared Spectroscopy (Shimadzu, 8400-S) using potassium bromide pellet technique. Hesperidin (≥80%) were mixed separately with stearic acid and tween 80 in 1:1 ratio and stored at 40°C for two weeks. The FT-IR spectra were then noted and studied.
Experimental design: 3² factorial design using Design expert (Version 9.2; Stat-Ease Inc., Minneapolis, Minnesota, USA) were used to carry out optimisation. The experiment were designed to study the effect of two independent variables namely lipid concentration and surfactant concentration on particle size, zeta potential and drug content [14]. The layout of the experimental design is shown in (Table 2).
Independent Variables
Levels
-1
0
+1
Lipid concentration (grams)
5
10
15
Surfactant concentration (grams)
2
4
6
Dependent Variables
Unit
Constrains
Particle Size
nm
maximize
Zeta Potential
mV
maximize
Drug Content
%
maximize
Table 2: The layout of experimental variable.
Characterization of SLN dispersion
Particle size and zeta potential: The particle size analysis and zeta potential of the selected formulation was performed using Malvern Zetasizer NanoZS90 (Malvern Instruments, Worcestershire, UK) and laser diffraction with beam length 2.40mm, range lens of 300 RF mm, and at 14.4% obscuration. For zeta potential analysis the samples were diluted in 1:10 ratio. Each value is the average of 3 measurements.
Drug content: Content of hesperidin in hesperidin SLN dispersion was determined by firstly washing a known quantity of dispersion with 0.1N sodium hydroxide solutions. The residue after complete drying was dissolved in known quantity of dimethyl formamide. Absorbance was measured after filtering the solution through a 0.45 micron membrane filter at 286 nm using UV- visible double beam spectrophotometer and % drug content was calculated.
Scanning electron microscopy: The surface morphology of hesperidin and formulation was determined using analytical scanning electron microscope (JSM-6360A, JEOL). The samples were lightly sprinkled on a double adhesive tape stuck to an aluminium stub. The stubs were then coated with platinum to a thickness of about 10Å under an argon atmosphere using a gold sputter module in a high- vacuum evaporator. Afterwards, the stub containing the coated samples was placed in the scanning electron microscope chamber.
Differential Scanning Calorimetry (DSC) [16,17]: DSC was performed on Mettler-Toledo DSC 821e (Columbus, OH) instrument and an empty standard aluminum pan was used as reference. DSC scans were recorded at heating rate of 10°C/min in temperature range 30-300 °C. DSC measurements were carried out on stearic acid, hesperidin, hesperidin SLN.
X-ray Powder Diffraction (XRPD) [18]: X ray diffractogram study was performed using Philips PAN analytical expert PRO X-ray diffractometer 1780. The radiation source used was CuKα with scanning rate (2hour/ min) of 5°C/min. X-ray diffraction measurements were carried out on hesperidin, stearic acid and hesperidin SLN.
In-vitro drug release
In-vitro drug release study was done on Hesperidin SLN (equivalent to 160 mg Hesperidin) contained in a dialysis bag (molecular cut-off 12000 Da). The study was carried out in USP Dissolution apparatus (Type II) at 100rpm, using 900ml of dissolution medium 0.1N HCl (2h) followed by Phosphate buffer 6.8 pH at 37±2°C upto 12 hours. Aliquots were withdrawn hourly till 12h and equal volume of fresh dissolution medium was replaced. The sample were filtered through 0.2μm filter and analysed spectrophotometrically at 286nm [19,20].
Preparation and evaluation of hesperidin SLN dispersion gel: Sodium Carboxy Methyl Cellulose (SCMC) gel (5%) was prepared by dispersing 5 gm SCMC in 85 gm distilled water and allowing it to hydrate for 2h followed by addition of 10 gm of glycerol. Hesperidin SLN dispersion (4%) was mixed with equal weight of gel to render gel containing 2% Hesperidin [21,22]. The gel was further evaluated for viscosity and spreadability. Brookfield digital viscometer, equipped with a T-Bar spindle coded S-92 was used to determine viscosity (cp) of the formulations. The viscosity was measured at 10 rpm after 30s. Measurements were performed at ambient temperature and in triplicate. Texture analyser (CEB Texture analyser, Brookfield Engineering Labs, Inc., Model Texture Pro CT V1.4 Build 17) was used to determine the spreadability of hesperidin SLN gel.
Ex-vivo permeation study: Excised skin of rat was cleansed and hair along with the sub dermal fat and fascia was removed. Then the skin was hydrated for one hour and then mounted on a Franz diffusion cell with diffusion area 3.14cm2 and 7ml cell volume with stratum corneum facing upwards. The receptor compartment was filled with phosphate buffer pH 7.4 and temperature was maintained at 37°C ± 0.5 under constant magnetic stirring. A dose of 320 mg of 2% hesperidin SLN gel was applied to the membrane in the donor compartment and covered with aluminium foil so as to prevent drying out [23,24]. Aliquots were withdrawn at predetermined time intervals over a period of 8h and analyzed spectroscopically at 286nm.
Pharmacodynamic study: Pharmacodynamic study was carried out on male Wistar rats weighing 180-220 gm. The experimental protocol was approved by the Institutional Animal Ethical Committee constituted as per the rules of the Committee for the Purpose of Control and Supervision of Experiments on Animals, India (CPCSEA) [25].
Induction of arthritis: Arthritis was induced by a single injection of 0.1ml Complete Freund’s Adjuvant (CFA) containing heat killed and dried Mycobacterium tuberculosis into subplantar region of the right hind paw on day one. Animals were divided into five groups with six rats in each group. Treatment was initiated after the onset of arthritis (day 7) and continued once daily until 28th day of the experiment [26]. The standard group was treated with oral dose of chloroquine (dose equivalent to 15mg). To the test group 1 Hesperidin SLN dispersion was given orally (dose equivalent to 160mg/kg). Hesperidin SLN gel with dose equivalent to 160mg/kg were applied topically to right hind paw of test group 2 as depicted in (Table 3).
Group
Treatment
Dose Administered
Normal
-
-
Control
-
-
Standard
Chloroquine dispersion
Dose equivalent to 150mg
Test 1
Hesperidin SLN dispersion (oral)
Dose equivalent to 160 mg
Test 2
Hesperidin SLN gel ( topical)
Dose equivalent to 160mg
Table 3: Experimental protocol for Pharmacodynamic study.
Evaluation parameters [26-29]
Measurement of hind paws volume: Paw volumes of both the right hind limbs were recorded from day 7 to 28 at 4 day interval using water displacement plethysmometer.
Radiological analysis: On day 28, animals were anesthetized with anesthetic ether. Radiographs of the adjuvant injected hind paws were taken with X-ray instrument. The film focus distance was 60 inches and the machine was operated at 43kV peak, 2ma. The X-ray images of the adjuvant-injected limb of the rat were evaluated for radiographic changes and were graded as follows (Table 7).
Group
Degree of erosion
Joint space narrowing
Bone destruction
Normal
0
0
0
Control
++++
++++
++++
Standard
+++
+++
+++
Hesperidin (Oral)
+ +
+ ++
+ +
Hesperidin (Topical)
+ +
+ +
+ +
0: no abnormality detected, +: damage changes up to 25 %, ++: damage changes up to 50 %, +++: damage changes up to 75 %, ++++: damage changes more than 75 %.
Table 7: X-ray examination results.
1) Periosteal reaction (0-3; none, slight, moderate, marked)
2) Erosions
3) Joint space narrowing
4) Joint space destruction
Histopathology study: Morphological analysis of histological sections of knee joints is essential for quantifying degree of joint damage and drug efficacy in animal model. Rats were sacrificed on the 29th day of the study by ether anesthesia. Knee joints were removed and fixed for 4 days in 4% formaldehyde. After decalcification in 5% formic acid, the specimens were processed for paraffin embedding tissue sections and stained with haematoxyllin and eosin.
Histopathological changes were scored using following parameters (Table 8).
Group
Degree of erosion
Periosteal thickening
Infiltration of cells
Connective tissue proliferation
Normal
0
0
0
0
Control
++++
++++
++++
++++
Standard
+++
+++
+++
+++
Hesperidin (oral)
++
+++
++
+++
Hesperidin
(topical)
++
++
++
++
0: no abnormality detected, +: damage changes up to 25%, ++: damage changes up to 50%, +++: damage changes up to 75%, ++++: damage changes more than 75%.
Table 8: Results of histopathology of joints.
- Infiltration of cells
- Bone erosion
- Joint space narrowing
- Connective tissue proliferation.
- Periosteal reaction
Analysis of tumor necrosis factor- alpha (TNF-α) using ELISA [30]: The levels of TNF-α in the synovial fluid sample were measured using commercially available ELISA kits that specifically recognize the rat cytokines according to the manufacturer’s instructions. Briefly, 100μl aliquots of sample were pipetted into the wells of a microtiter plate pre-coated with an antibody specific for rat TNF-α and incubated for 2.5h at room temperature. After washing, a different biotinylated anti-rat TNF-α antibody was added and incubated at ambient temperature for 1h. Streptavidin-peroxidase was added and incubated for 30min. After a third incubation and washing to remove all unbound enzyme, colour was developed by addition of stabilized chromogen (tetramethylbenzidine), a stop solution added and the intensity of the coloured product quantified spectrophotometrically at 425nm.
Result and Discussion
Compatibility studies
The IR spectra depicts drug peak 3480cm-1(O-H stretch aromatic hydroxyl group), 1650cm-1(C=C aromatic bond) and 1750cm-1 (C=O Aromatic group) (Figure 1). The spectra for combinations were seen to retain all these peaks and no new peaks were detected leading to conclusion that there was no interaction between Hesperidin and excipients.
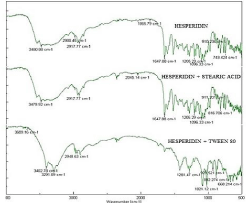
Figure 1: FT IR spectra of drug, drug+ Stearic acid, drug+ Tween 80
respectively.
Preparation hesperidin SLN dispersion
Hesperidin did not have good solubility in any of the lipids normally used hence a co solvent DMF was used to achieve solubility. The stearic acid was used as it is seen that negatively charged lipid particles are taken up preferentially in lymphatic system. Hesperidin SLN dispersion was prepared using emulsification- probe sonication method. Since it is observed that lipid and surfactant concentration are two most important factors governing performance properties of SLN namely particle size, drug content and zeta potential a two 32 full factorial design was employed to yield a optimized product [31]. The factor combination and performance properties of trial batches are listed in (Table 4).
Formulation code
Lipid concentration (grams)
Surfactant
Concentration
(grams)
Particle size
(nm)
Zeta Potential
(mV)
Drug Content
(%)
1
5
2
212
-15.6
16.18
2
10
2
301
-17.8
20.81
3
15
2
356
-19.2
32.2
4
5
4
206
-12.5
16.3
5
10
4
258
-17.1
38.2
6
15
4
348
-18.9
35.5
7
5
6
192
-9.95
16.6
8
10
6
239
-16.5
28.6
9
15
6
328
-18.4
39.4
*Mean ± SD, n=3
Table 4: Trial runs of 32 Experimental design for optimization of Hesperidin SLN.
Experimental design
The Design Expert 9.0 was used to create statistical model to predict the relationship between response and variables and to illustrate the interaction between variables.
Influence of independent variable on particle size
Particle size=+266+70^* A-18.33^* B+2^* AB+8^* A^2+3.76^*B^2
The particle size of formulations of trial run showed particle size in the range of 192-356 nm. An increase in particle size was observed with increase in lipid concentration of the formulation as viscosity of suspended lipid in emulsion increases and the homogenization has less effect in particle size reduction. The particle size decreases with increase in tween 80 concentration as low surfactant concentration was unable to provide protective layer around the particles thus causing aggregation. Effect of lipid was more dominant over that of tween 80 and hence an interaction between the two indicates some increase in particle size in (Figure 2a).
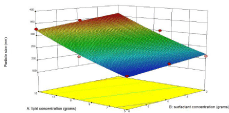
Figure 2a: Influence of independent variables on zeta potential.
Influence of independent variable on zeta potential
zeta potential=-17.08-3.08^* A+1.29^* B-1.21^* AB+1.38^*A^2-0.075^* B^2
The zeta potential of formulations of trial run was in the range of -9.95 to -19-2 mV. The increase in lipid concentration causes increase in zeta potential in negative direction which is because of the negative charges of stearic acid while tween 80 being a non-ionic surfactant exerted feeble effect and interaction between the two decreased zeta potential slightly in (Figure 2b).
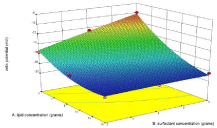
Figure 2b: Influence of independent variables on zeta potential.
Influence of independent variable on % drug content
% Drug Content=+32.12+9.67^* A+2.57^* B+1.69^* AB-3.17^*A^2-4.37^* B^2
The drug content of formulations of trial run showed particle size in the range of 16.18 to 39.4%. The increase in lipid concentration led to increased drug content as it cause emulsion droplets with higher viscosity and the migration of drug in surrounding dispersion medium is prevented. The percent drug content increases, highest drug content was found with highest amount of lipid. Since the surfactant used is water soluble it caused decrease in drug content from the dispersion. But the effect so produced was very negligible as depicted in (Figure 2c).
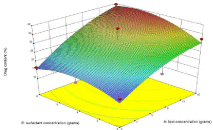
Figure 2c: Influence of independent variables on % drug content.
The R2, for all response variables demonstrated good fit of the model and the F values suggested that the model was significant (p<0.05) as depicted in (Table 5) The P value below 0.005 and higher F value for all the 3 models indicates significant model system. Also, the R2 values manifest the exceptional accession between the formulation variables and response framework. Adequate precision denotes signal to noise ratio and its value greater than 4 demonstrates minimum noise. Thus, these models can be used to predict the values of the response framework at selected values of formulation variables within design space.
Sr. no
Outcomes
Particle size
Zeta Potential
% Drug Content
1
F value
30.28
35.74
4.54
2
P value
<0.05
<0.05
<0.05
3
R2 value
0.9806
0.9835
0.8833
4
Adequate precision
14.987
16.358
5.540
Table 5: ANOVA outputs of 32 factorial design for optimization of hesperidin SLN dispersion.
The optimized formulae suggested by software as per the criteria provided in table are listed in (Table 6). The % error was less and thus prognostic ability of model generated is established.
Formulation code
Composition of optimized formulation
Response
Predicted value
Actual Value
% Error
Desirability
A
B
OF1
10.264
2.843
Y1
280.387nm
279nm
0.49
0.707
Y2
-17.977mV
-17.86mV
0.65
Y3
29.619%
29.60%
0.03
OF2
10.259
2.823
Y1
280.491nm
280.48nm
0.003
0.707
Y2
-17.988mV
-17.976mV
0.06
Y3
29.531%
29.52%
0.03
Table 6: Checkpoint for optimization, actual, predicted value and % error (n=3, Mean±SD).
Characterization of SLN dispersion
Particle size, zeta potential and drug content: The particle size, zeta potential and drug content of the optimised batch of hesperidin SLN dispersion was found to be 279nm, 17.86mV and 29.60 % as depicted in (Figure 3).
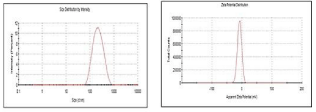
Figure 3: Particle size and zet potential analysis.
Scanning electron microscopy: SEM images in (Figure 4) revealed presence of almost spherical morphology of the SLN this is beneficial upon oral administration as sphericity is known to improve lymphatic uptake [32].
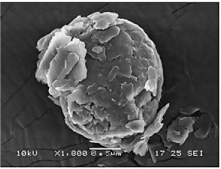
Figure 4: SEM analysis.
Differential Scanning Calorimetry (DSC): The thermogram revealed a single endothermic peak for stearic acid (G2) at 66°C. Hesperidin also produced a single endothermic peak (G3) at 260°C. Hesperidin SLN showed two endothermic peak one at (G1) 63°C and other at 250°C. The thermal properties remained unchanged in the formulation as depicted in (Figure 5).
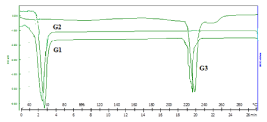
Figure 5: DSC analysis of G1- formulation, G2- Stearic acid and G3-
Hesperidin.
X-ray diffractometry: X-Ray diffractogram in (Figure 6) of hesperidin showed sharp peaks due to crystalline nature of drug but the SLN due to trapping of drug in molecular form was showed a halo pattern indicating loss of crystallinity.
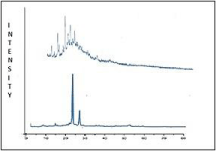
Figure 6: XRD of hesperidin and Hesperidin SLN.
Preparation and evaluation of hesperidin SLN dispersion gel: The hesperidin SLN gel found to have a viscosity and spreadability of 3468 cps and 23.01g/sec respectively.
In-vitro drug release: Owing to higher release of hesperidin from hesperidin SLN in 6.8 pH as compared to 0.1N hydrochloric acid, the comparison of hesperidin drug and hesperidin SLN was done in simulated gastric fluid for 2 hours and further in simulated intestinal fluid. Hesperidin in 12 hours showed a release of 15.11% and hesperidin from SLN formulation showed a release of 51.11%. Hesperidin from formulation had a sustain release. The release followed Peppas model with n = 0.998 and k = 7.349 which means an anomalous release mechanism combining diffusion and erosion (Figure 7).
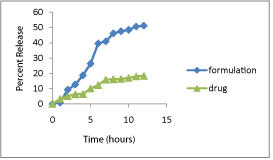
Figure 7: In-vitro drug release study.
Ex-vivo permeation Study: The experimental conditions were maintained at 37°C as the rate as extent of skin absorption from gel is temperature-dependent [24]. The % localisation of drug in skin was found to be 65.8% and the flux was calculated for hesperidin SLN gel was found to be 0.63μg/cm2.
Pharmacodynamic study
Induction of arthritis: CFA induced arthritis model CFAinduced arthritis is the mostly used chronic test model in which the clinical as well as the pathological changes are comparable with those seen in human rheumatoid arthritis. Treatment was initiated after onset of arthritis (day 7) and continued until day 28.
Evalution parameters
Measurement of hind paws volume: Paw volumes of the right hind limbs were recorded from day 7 to 28 at 4 day interval using plethysmometer. A significant decrease in paw volume was seen in both the treatment and standard groups. Hesperidin SLN gel (test 2) showed highest reduction in paw volume when compared with other groups. Statistical analysis by two-way ANOVA depicted in (Figure 8) followed by Bonferroni multiple comparison test using Graph Pad Prism Software 5.03 was applied to the above data. *P<0.0001 and **P<0.0001 attributed all values to be significant. The results were the mean of triplicate measurement. This is because chloroquine was administered as simple suspension whereas earlier reports from our lab have showed significant outcome when chloroquine was administered as SLN [11].
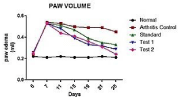
Figure 8: Effect of hesperidin SLN on paw edema.
Radiological analysis: Radiographs are necessary to determine true remission and accurate evaluation of disease status. Reduced bone formation and increased resorption are the cause for bone loss in CFA induced arthritis [26]. Radiographs taken on 28th day i.e. post treatment were compared for degree of erosion, joint space narrowing and bone destruction. As per the results obtained, test group 2 i.e. Hesperidin SLN gel were most effective among others. Degree of erosion and bone destruction was upto 50% in test 2 and test 1 when compared with arthritic control and standard depicted in (Figure 9). But test 1 group showed more damage in terms of joint space narrowing than test 2 group. Hence it can be concluded that hesperidin SLN gel showed the best result among all other groups. There was a significant clearance in paw volume which may be due to COX-2 inhibition activity OF hesperidin drug.

Figure 9: X-ray studies.
Histopathology study: Photomicrographs of joint section obtained after Haematoxyllin and eosin staining are documented in (Figure 10). As per results, test 2 group i.e. hesperidin SLN gel was the most effective among other groups. Hesperidin SLN gel showed minimal infiltration of inflammatory cells and connective tissue proliferation as compared to test 1 group which showed moderate infiltration of inflammatory cells and connective tissue proliferation. Periosteal reaction was majorly seen in arthritic control group.
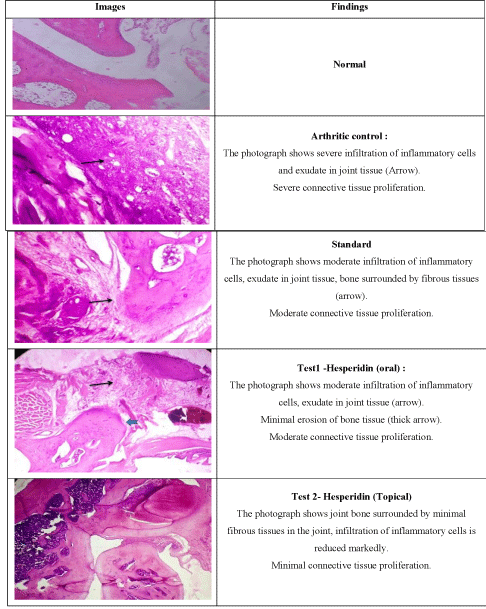
Figure 10: Histopathology of knee joints.
Analysis of tumor necrosis factor- alpha (TNF-α) using ELISA: The synovial fluid was obtained by homogenization of the limbs was subjected to TNF-α ELISA assay. As per the protocol given in section the antigen from the sample was immobilized on the titre well plate. After immobilization the detection antibody was added forming a complex with antigen. This antibody was covalently linked to a specific enzyme, HRP Streptavidine through bioconjugation. The plate was developed by the addition of enzyme substrate which reacted with the enzyme producing blue colour. The reaction was halted by addition of stop solution thus producing yellow colour which was analyzed as depicted in (Figure 11). The absorbance was measured at 425nm using plate reader. Test 2 group i.e. hesperidin SLN gel showed the least concentration of TNF-α. The results were the mean of quintuplicate measurement.
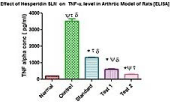
Figure 11: Level of TNF-α in the synovial fluid.
(*)- Comparison of control with other groups, (Ψ)- Comparison of standard with
other groups, (τ)- Comparison of test 1 with other groups, (δ)- Comparison of
test 2 with other groups.
Two ways ANOVA followed by Bonferroni multiple comparision test using GraphPad Prism Software (Figure 8) was used to statistically analyze the result. *P< 0.001 (when compared with arthritic control), ΨP< 0.05 (when compared with standard) attributed these values to be significant. Since the concentration of TNF-α for test 1 and test 2 group were almost similar therefore P value was found to be non significant, τP> 0.05 , δP>0.05( when test 1and 2 were compared with other groups respectively). Hence when unpaired t test was applied to test 1 and test 2, a significant result was obtained, P<0.0001 (Figure 12).
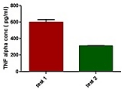
Figure 12: Unpaired T- test to compare test 1&2.
Conclusion
It can be seen that when hesperidin is administered in SLN carrier orally and topically, result showed a significant disease modifying action over Chloroquine dispersion. From this study it was proved that topical hesperidin SLN gel showed better action than hesperidin oral dispersion. However effects of topical and oral administration did not differ significantly. Hence hesperidin SLN can be used to enhance activity of hesperidin for the treatment of rheumatoid arthritis.
References
- Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology. 2012; 5: 3-11.
- Fishman P, Yehuda SB. Rheumatoid Arthritis: History, Molecular Mechanisms and Therapeutic Applications. A3 Adenosine Receptors from Cell Biology to Pharmacology and Therapeutics. Springer Science+Business Media B.V. P.A. Borea (ed). 2010; 291-299.
- Mamum MAA, Hosen MJ, Islam K, Khatun A, Alam MM & Al-Bari MAA. Tridax procumbens flavonoids promote osteoblast differentiation and bone formation. Biological Research. 2015; 48: 65.
- Yocum DE. T Cells: Pathogenic Cells and Therapeutic Targets in Rheumatoid Arthritis. Seminars in Arthritis and Rheumatis. 1999; 29: 27-35.
- MClnnes IB, Schett G. The Pathogenesis of Rheumatoid Arthritis. The New England Journal of Medicine. 2011; 365: 23.
- Li R, Li J, Cai L, Hu CM, Zhang L. Suppression of adjuvant arthritis by hesperidin in rats and its mechanisms. Journal of Pharmacy & Pharmacology. 2008; 60: 221-228.
- Joseph B, Raj SJ. Pharmacognostic and phytochemical properties of aloe vera linn–an overview. International Journal of Pharmaceutical Sciences Review and Research. 2010; 4: 106-110.
- List of Supplements. 2015.
- Majumdar S, Srirangam R. Physicochemical Characteristics and In Vitro Ocular Tissue Permeability of Hesperidin: a Natural Bioflavonoid. Pharmaceutical Research. 2009; 26: 1217-1225.
- Bilia AR, Isacchi B, Righeschi C, Guccione C, Bergonzi MC. Flavonoids Loaded in Nanocarriers: An Opportunity to Increase Oral Bioavailability and Bioefficacy. Food and Nutrition Sciences. 2014; 5: 1212-1227.
- Bhalekar MR, Upadhaya PG, Madgulkar AR. Fabrication and efficacy of Chloroquine nanoparticles in CFA- induced arthritic rats using TNF-α ELISA kit. European Journal of Pharmaceutical Science. 2016; 84: 1-8.
- Zhao J, Liu T, Xu F, You S, Li C, Gu Z. Antiarthritic effects of total flavonoids from Juniperus sabina on complete freund’s adjuvant induced arthritis in rats. Pharmacognosy Magazine. 2016; 12: 178-183.
- Jawahar N, Meyyanathan SN, Reddy G, Sood S. Solid lipid nanoparticles for oral delivery of poorly soluble drugs. Journal of Pharmaceutical Sciences and Research. 2012; 4: 1848-1855.
- Gambhire M, Bhalekar M, Shrivastava B. Bioavailability assessment of simvastatin loaded solid lipid nanoparticles after oral administration. Asian Journal of Pharmaceutical Sciences. 2011; 6: 251-258.
- Raouf A, Hammud K, Mohammed J. Qualitative and quantitative determination of folic acid in tablets by FTIR spectroscopy. International Journal of Advance in Pharmacy, Biology & Chemistry. 2014; 3: 773-780.
- Gajre B, Dalwadi C, Patil R. Formulation and optimization of itraconazole polymeric lipid hybrid nanoparticles (Lipomer) using box behnken. DARU Journal of Pharmaceutical Science. 2015; 3: 23-33.
- Jieheng Y, Qun W, Xuefeng Z, Na Z. Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. International Journal of Pharmaceutics. 2008; 352: 273-279.
- Vennishetty Y, Komuravekki R, Kuncha M and Sistla R. Increased brain uptake of docetaxel and ketoconazole loaded folate grafted solid lipid nanoparticles. Nanomedicine. 2013; 9: 111-121.
- Smith A, Hunneyball IM. Evaluation of polylactid as a biodegradable drug delivery system for parenteral administration. International Journal of Pharmaceutics. 1986; 30: 215-230.
- MuÈhlen A, Niehus H, Mehnert W. Atomic force microscopy studies of solid lipid nanoparticles. Pharmaceutical Research.1996; 13: 1411.
- Das MK, Ahmed AB. Formulation and evaluation of rofecoxib gel for topical application. Acta Poloniae Pharmaceutica. 2007; 63: 461-467.
- Carter SJ. Cooper and Gunn’s Dispersing of Pharmaceutical Students. 12th ed. Delhi: CBS Publishers and Distributors. 2000; 192-199.
- Guidance for industry nonsterile semisolid dosage forms. Scale-Up and Post approval Changes (SUPAC): Chemistry, manufacturing, and controls; in-Vitro release testing and in-Vivo bioequivalence documentation.
- A report by Dr Walter Diembeck. Head of In Vitro Biocompatibility/Screening Department, BDF Beiersdorf AG. ‘Using in vitro skin absorption and penetration testing as an alternative to animal testing’.
- Newbould BB. Chemotherapy of arthritis induced in rats by injection of mycobacterial adjuvant. Brazillian Journal of Pharmacoogyl Chemotherapy. 1963; 21: 127-137.
- Snekhalatha U, Anburajan M, Venkatraman B, Menaka M. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model Comparison of thermography and histopathology. Zeitschrift fur Rheumatologie. 2013; 72: 375-382.
- Ekambaram. Evaluation of antiarthritic activity of Strychnos potatorum Linn seeds in Freund’s adjuvant induced arthritic rat model. BMC Complementary and Alternative Medicine. 2010; 10: 56.
- Bhalekar MR, Upadhaya PG, Madgulkar AR, Nalawade SD, Kshirsagar SJ. Anti-rheumatic activity of chloroquine-SLN gel on wistar rats using Complete Freund’s Adjuvant (CFA) model. Indian Journal of Rheumatology. 2015: 58- 64.
- Woode E, Boakye GE, Danquah C. Anti-arthritic effects of Palisota hirsute K Schum. Leaf extract in Freund’s adjuvant- induced arthritis in rats. International Journal of Pharmacology. 2000; 5: 181-190.
- Barton N, Stevens D, Hughes J. Demonstration of a novel technique to quantitatively assess inflammatory mediators and cells in rat knee joints. Journal of Inflammation. 2007; 4: 13.
- Dhoranwala KA, Shah P and Shah S. Formulation Optimization of Rosuvastatin Calcium- Loaded Solid Lipid Nanoparticles by 32 Full-Factorial Design. Nano World Journal. 2015; 1: 110-120.
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery. 2010; 9: 615-627.
