Abstract
Rheumatoid Arthritis (RA) is a common autoimmune inflammatory disease that is causing trouble to nearly 70 million peoples throughout the world. Treatment of RA has gone through a series of improvements in therapeutic effects with Methotrexate (MTX), Prednisolone Phosphate (PLP), and many others. On the basis of these effective therapeutic agents, development of polymer nanotherapeutics leads us to a new era of treating RA, which not only optimize the pharmacokinetics via controlled drug delivery, but also up-regulate the distribution and accumulation of drugs at the inflamed sites. In this article, we briefly illustrate the polymer nanocarriers employed in passive or active targeting drug delivery systems, and compare their distinct advantages.
Keywords: Polymer; Nanocarriers; Targeting; Controlled drug delivery; Rheumatoid arthritis therapy
Introduction
Rheumatoid Arthritis (RA) is a chronic autoimmune disorder induced by several factors, such as genetic sensitivity, environment factor, infection, sexual hormones, etc. [1-3]. A wide range of cell types and cytokines accounts for the pathological process of RA. Currently, surgery and drugs are the two most frequently used treatment strategies for RA. Local surgery, represented by synovectomy and joint replacement, cannot fundamentally resolve RA as it is a systemic disease. As for therapeutic agents, Disease-Modifying Anti- Rheumatic Drugs (DMARDs), Glucocorticoids (GCs), Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), and biological agents are proved effective in clinical practice, and some even remarkably improve the therapeutic effects. However, long-term systemic administration of the pharmaceuticals above will inevitably lead to systemic toxicity and infection. Besides, short half-time and high costs of these drugs also impede their wide application.
Recently, advances in the exploration of polymer nanotherapeutics have provided a new approach to the difficulties in treating RA patients, and the nanodrugs turn out an optimum choice for therapy of RA. Polymer nanovehicles potentially protect drugs against biodegradation in the blood circulation [4], in which case they achieve prolonged circulation and sustained release. Moreover, therapeutic agents are selectively delivered to and accumulated in the inflamed sites by passive or active targeting approach.
Abnormal vessels and recruitment of inflammatory cells are two most remarkable features of RA, and thus are often utilized in selective drug delivery. Passive targeting approach is based on the Enhanced Permeability and Retention (EPR) effect, first discovered in 1986 [5]. It was determined that the nanovehicles with diameters of about 100 nm were most outstanding in anti-inflammatory capability through intravenous administration on experimental arthritis models [6]. Moreover, the targeting ligands on the surface of polymer nanoformulations will enhance the selective accumulation of drugs in the lesion sites through ligand-receptor interaction [7,8].
Polymer nanotherapeutics alleviate rheumatoid arthritis
Hyaluronic Acid (HA)-conjugated methotrexate (MTX) reduced the proliferation of fibroblast-like synoviocytes (FLs) in vitro [9], and arthritis symptoms were consequently alleviated [9-11]. Apart from prolonging the local circulating time of GCs, nanocarriers can also reduce doses and frequency of drug use [12,13]. 30% decrease in arthritic inflammation was demonstrated at the first day post administration and this effect sustained for one week [12]. In order to control the release profile of therapeutic agents, researchers modified nanovehicles with new properties. In one study, they prepared polymerizable and hydrolytically cleavable dexamethasone (DEX) derivatives that covalently encapsulated in the core-crosslinked Polymer Micelles (PMs). Controlled release can be realized by changing the oxidation degree of thioether [14]. Being a representative NSAIDs, Indomethacin (IND) loaded in PMs possessed sustained antiinflammation efficacy and simultaneously reduced undesirable effects [15-18]. It was found that this formulation maintained the effective concentration of IND and decreased the sera level of tumor necrosis factor-a (TNF-a) and interleukin-6 (IL-6) in experimental arthritis models. Besides, the bioavailability of IND was largely promoted with nanotherapeutics, which meant that a lower dosage of IND was sufficient to perform the same therapeutic efficacy that can only be achieved by a much higher dosage of free drugs [19,20]. Recently, biological agents arose as a novel therapy pattern for treatment of RA. Negatively charged temperature-sensitive amphiphilic polyelectrolyte (succinylatedpullulan-graft-oligo (L-lactide); SPL) was combined with positively charged Etanercept (ETA; antibody of TNF-a) into a temperature-modulated noncovalent interaction controllable complex that featured prolonged pharmacokinetics [21]. Moreover, hyaluronate-gold nanoparticle/tocilizumab (HA-AuNP/TCZ) was developed and researched [22], and further revealed that AuNP was recognized by Vascular Endothelial Growth Factor (VEGF) in RA mice [23]. In another study, relative inflammation indices, namely clinical arthritic sore, radiographic sore, and pathological evaluation, were found significantly suppressed, confirming that AuNP was not only a nanovehicle for drug delivery, but also acted as a therapeutic agent by specific binding to VEGF in the treatment of RA [24].
Several other therapeutic agents also made much difference to the treatment of RA. AuNP-Gal-1 was determined to specifically reduce CD4+ T cells through intra-articular administration, resulting in the reduced level of pro-inflammatory cytokines and alleviated symptoms in Collagen-Induced Arthritis (CIA) [25]. More recently, TNF-Related Apoptosis Inducing Ligand (TRAIL) has come into light as an emerging anti-inflammation drug [26,27]. In order to extend the half-life of TRAIL, positively charged TRAIL and HA were combined into a nanocomplex, which achieved satisfactory outcome [28]. Later, Photosensitize (PS) was employed in the nanotherapeutics of RA as Photodynamic Treatment (PDT). PEGliposomes- encapsulated temoporfin, a subset of PS, showed the highest accumulation in arthritic joints, and therefore PDT elicited remarkable anti-inflammatory activity [29]. In a more recent study, hydrogel based on the MTX-loaded Nanostructured Lipid Carriers (NLCs) and Chemical Enhancer (CE) showed significantly improved anti-inflammatory ability characterized by sharp reduce in the severity of inflammation (Figure 1) [30]. In short, passive targeting delivery systems for nanotherapeutics of RA are advantageous in the following aspects: 1. Prolonged pharmacokinetics; 2. Enhanced accumulation in inflammation tissues with improved therapeutic efficacy; 3. Reduced side effects owing to decreased biodistribution in normal organs.
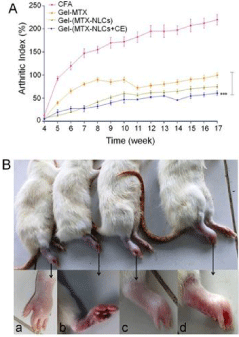
Figure 1: (A) Calculation of arthritis index of various MTX formulations, and
(B) assessment of inflammation severity of experimental rat. a, b, c, and d are
the paw images after the treatment of gel-(MTX-NLCs), gel-(MTX-NLCs+CE),
gel-MTX, and complete Freud’s adjuvant control (without treatment),
respectively [30].
While passive targeting nanomedicine undoubtedly outweighs traditional methods, active targeting strategy, on the other hand, can further optimize the targetability of nanovehicles by using ligands on the surface that specifically bind to receptors with high affinity. In most cases, these receptors are highly or exclusively expressed on targeted cells in particular microenvironment.
Macrophages, or rather, activated macrophages under inflammatory circumstance, are essential in the pathology of RA [31], mainly because a series of specific receptors, for instance, Folate Receptor (FR) [32,33] and Scavenger Receptor (SR) [8,34], are highly expressed on the surfaces of activated macrophages. It was demonstrated that the Folic Acid (FA)-conjugated EC20 (a folate-linked imaging agent) was found highly accumulated in the arthritic joints [35]. In our previous work, we prepared a dextrangraft- methotrexate/folate (Dex-g-MTX/FA) prodrug (Figure 2), which showed excellent targetability toward activated macrophages in vitro and was mostly located at the affected joints. Systemic administration of Dex-g-MTX/FA effectively reduced the level of pro-inflammatory cytokines and also obviously relieved the arthritis symptoms [7]. Other than that, several other FR-targeted conjugates, such as FA-PEG-poly-(amidoamine) (G3.5 PAMAM) [36] and FAMTX- PAMAM conjugate [37], were prepared and proved effective as nanotherapeutics of RA.
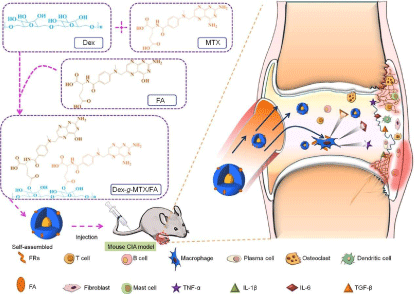
Figure 2: Schematic illustration of Dex-g-MTX/FA in treatment of CIA [7].
SRs belong to macrophages surface glycoproteins, which specifically recognize the oxidized Low-Density Lipoprotein (LDL), serum albumin, and polyanionic macromolecules [38-40]. In this regards, Dextran Sulfate (DS), a hydrophilic block, was adopted as an active target for it specifically recognize SRs through ligand-receptor interaction. In one study, DS was combined with Polycaprolactone (PCL) into DS-b-PCL, which exhibited improved biodistribution in the hind paws of CIA mice after each systemic administration, while little was detected in wild type mice [34]. In our group, a SR-targeted DS-MTX conjugate was synthesized for targeted treatment of CIA [8]. DS-g-MTX selectively recognized activated macrophages in vitro and in vivo, and selectively accumulated in the inflamed joints. Moreover, DS-g-MTX induced the significant alleviation of synovitis and protection of articular cartilage by inhibiting the expression of pro-inflammatory cytokines after intravenous injection. Overall, the activated macrophage-targeted prodrug showed great potential for targeted treatment of RA.
Similar to activated macrophages, Vascular Endothelial Cells (VECs) show distinct features from normal cells, such as the expression of adhesion molecules (e.g., avβ3-integrin and E-selectin), which makes VECs a potential target for active targeting nanotherapeutics of RA. Based on this property, a series of studies was carried out for further investigation of the avβ3-integrin-targeted nanovehicles in experimental arthritic models, achieving increased delivery to the inflammatory lesion, and stronger anti-inflammatory capability [19,41-43]. As a specific ligand of avβ3-integrin, Arginine-Glycine- Aspartic Acid (RGD) was introduced in poly-(DL-lactic-co-glycolic acid) (PLGA) Au half-shell NP loading MTX. Near-Infrared (NIR) absorbance image confirmed that stronger absorbance intensity of the active targeting NP was demonstrated in inflamed joints compared with non-inflamed joint and non-targeted NPs [44].
As a member of selectin family, E-selectin is over expressed on neovascular [45] as well as endothelial cells under inflammatory conditions [46]. It was reported that sialyl Lewis X (SLX; a sugar chain) could recognize and bind to E-selectin, and was utilized for active targeting drug delivery [47]. Therefore, it was indicated that interaction between SLX and E-selectin was regulated in a receptormediated manner [48].
Conclusion
RA, a systemic autoimmune disease, is associated with various pathogenesis. Polymer nanotherapeutics for RA as a novel therapeutic approach comes with several advantages compared with traditional treatment, such as highly selective accumulation, reduced systemic toxicity, decreased treatment frequency, controlled release of drugs, and so forth. All the virtues mentioned above to a large extent avert the drawbacks brought by traditional medicine and surgery. Particularly, the active targeting strategy of polymer nanotherapeutics further improves the therapeutic efficacy. Conclusively, polymer nanotherapeutics possesses great potential for targeted treatment of RA.
References
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003; 423: 356-361.
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis, Lancet .2010; 376: 108-109.
- McInnes IB, O’Dell JR. State-of-the-art: rheumatoid arthritis. Annals of the Rheumatic Diseases. 2010; 69: 906-1898.
- Ulbrich W, Lamprecht A. Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. Journal of the Royal Society Interface. 2010; 7: 55-66.
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research. 1986; 46: 6387-6392.
- Ishihara T, Kubota T, Choi T, Higaki M. Treatment of Experimental Arthritis with Stealth-Type Polymeric Nanoparticles Encapsulating Betamethasone Phosphate. Journal of Pharmacology and Experimental Therapeutics. 2009; 329: 412-417.
- Yang MD, Ding JX, Zhang Y, Chang F, Wang JC, Gao ZL, et al. Activated macrophage-targeted dextran-methotrexate/folate conjugate prevents deterioration of collagen-induced arthritis in mice. Journal of materials Chemistry B. 2016; 4: 2102-2113.
- Yang M, Ding J, Feng X, Chang F, Wang Y, Gao Z, et al. Scavenger Receptor-Mediated Targeted Treatment of Collagen-Induced Arthritis by Dextran Sulfate-Methotrexate Prodrug. Theranostics. 2017; 7: 97-105.
- Homma A, Sato H, Tamura T, Okamachi A, Emura T, Ishizawa T, et al. Synthesis and optimization of hyaluronic acid-methotrexate conjugates to maximize benefit in the treatment of osteoarthritis. Bioorganic & Medicinal Chemistry. 2010; 18: 1062-1075.
- Homma A, Sato H, Okamachi A, Emura T, Ishizawa T, Kato T, et al. Novel hyaluronic acid-methotrexate conjugates for osteoarthritis treatment. Bioorganic & Medicinal Chemistry. 2009; 17: 4647-4656.
- Shin JM, Kim SH, Thambi T, You DG, Jeon J, Lee JO, et al. A hyaluronic acidmethotrexate conjugate for targeted therapy of rheumatoid arthritis. Chemical Communications. 2014; 50: 7632-7635
- Higaki M, Ishihara T, Izumo N, Takatsu M, Mizushima Y. Treatment of experimental arthritis with poly-(D,-L-lactic/glycolic acid) nanoparticles encapsulating betamethasone sodium phosphate. Annals of the Rheumatic Diseases. 200; 64: 51132-51136.
- Hwang J, Rodgers K, Oliver JC, Schluep T. Alpha-methyl prednisolone conjugated cyclodextrin polymer-based nanoparticles for rheumatoid arthritis therapy. International Journal of Nanomedicine. 2008; 3: 359-371.
- Crielaard BJ, Rijcken CJF, Quan L, Van Der Wal S, Altintas I, Van Der Pot M, et al. Glucocorticoid-loaded core-cross-linked polymeric micelles with tailorable release kinetics for targeted therapy of rheumatoid arthritis. Angewandte Chemie - International Edition. 2012; 51: 7254-7258.
- Zhang JX, Yan MQ, Li XH, Qiu LY, Li XD, Li XJ, et al. Local delivery of indomethacin to arthritis-bearing rats through polymeric micelles based on amphiphilic polyphosphazenes. Pharmaceutical Research. 2007; 24: 1944- 1953.
- Zhang JX, Li SH, Li XH, Qiu LY, Li XD, Li XJ, et al. Physicochemical characterization, in vitro, and in vivo evaluation of Indomethacin-loaded nanocarriers self assembled by amphiphilic polyphosphazene. Journal of Biomedical Materials Research Part A. 2008; 86: 914-925.
- Zhang JX, Li XJ, Qiu LY, XH Li, Yan MQ, Yi J, et al. Indomethacin-loaded polymeric nanocarriers based on amphiphilic polyphosphazenes with poly (N-isopropylacrylamide) and ethyl tryptophan as side groups: Preparation. In vitro and in vivo evaluation. Journal Of Controlled Release. 2006; 116: 322-329.
- Bernardi A, Zilberstein AC, Jager E, Campos MM, Morrone FB, Calixto JB, et al. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. British Journal of Pharmacology. 2009; 158: 1104-1111.
- Che L, Zhou J, Li S, He H, Zhu Y, Zhou X, et al. Assembled nanomedicines as efficient and safe therapeutics for articular inflammation. International Journal of Pharmaceutics. 2012; 439: 307-316.
- Nagai N, Ito Y. Effect of Solid Nanoparticle of Indomethacin on Therapy for Rheumatoid Arthritis in Adjuvant-Induced Arthritis Rat. Biological & Pharmaceutical Bulletin. 2014; 37: 1109-1118.
- Jung YS, Park W, Na K. Temperature-modulated noncovalent interaction controllable complex for the long-term delivery of etanercept to treat rheumatoid arthritis. Journal of Controlled Release. 2013; 171: 143-151.
- Lee H, Lee MY, Bhang SH, Kim BS, Kim YS, Ju JH, et al. Hyaluronate-Gold Nanoparticle/Tocilizumab Complex for the Treatment of Rheumatoid Arthritis. ACS Nano. 2014; 8: 4790-4798.
- Tsai CY, Shiau AL, Chen SY, Chen YH, Cheng PC, Chang MY, et al. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis & Rheumatism. 2007; 56: 544-554.
- Arvizo RR, Bhattacharyya S, Kudgus RA, Giri K, Bhattacharya R, Mukherjee P. Intrinsic therapeutic applications of noble metal nanoparticles: past, present and future. Chemical Society Review. 2012; 41: 2943-2970.
- Huang YJ, Shiau AL, Chen SY, Chen YL, Wang CR, Tsai CY, et al. Multivalent structure of galectin-1-nanogold complex serves as potential therapeutics for rheumatoid arthritis by enhancing receptor clustering. European Cells & Materials. 2012; 23: 170-181.
- Mackay F, Kalled SL. TNF ligands and receptors in autoimmunity: An update. Current Opinion in Immunology. 2002; 14: 783-790.
- Tsokos GC, Tsokos M. The TRAIL to arthritis. The Journal of Clinical Investigation. 2003; 112: 131-137.
- Na SJ, Chae SY, Lee S, Park K, Kim K, Park JH, et al. Stability and bioactivity of nanocomplex of TNF-related apoptosis-inducing ligand. International Journal of Pharmaceutics. 2008; 363: 149-154.
- Hansch A, Frey O, Gajda M, Susanna G, Boettcher J, Brauer R, et al. Photodynamic treatment as a novel approach in the therapy of arthritic joints. Lasers in Surgery and Medicine. 2008; 40: 265-272.
- Garg NK, Singh B, Tyagi RK, Sharma G, Katare OP, Effective transdermal delivery of methotrexate through nanostructured lipid carriers in an experimentally induced arthritis model. Colloids and Surfaces B: Biointerfaces. 2016; 147: 17-24.
- Kinne RW, Stuhlmuller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages, Arthritis Research & Therapy. 2007; 9: 224.
- Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Advanced Drug Delivery Reviews. 2004; 56: 1205-1217.
- Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Accounts of Chemical Research. 2008; 41: 120-129.
- Kim SH, Kim JH, You DG, Saravanakumar G, Yoon HY, Choi KY, et al. Selfassembled dextran sulphate nanoparticles for targeting rheumatoid arthritis. Chemical Communications. 2013; 49: 10349-10351.
- Turk MJ, Breur GJ, Widmer WR, Paulos CM, Xu LC, Grote LA, et al. Folatetargeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis and Rheumatism. 2002; 46: 1947-1955.
- Chandrasekar D, Sistla R, Ahmad FJ, Khar RK, Diwan PV. Folate coupled poly (ethyleneglycol) conjugates of anionic poly (amidoamine) dendrimer for inflammatory tissue specific drug delivery. Journal of Biomedical Materials Research. 2007; 82: 103-192.
- Thomas TP, Goonewardena SN, Majoros IJ, Kotlyar A, Cao Z, Leroueil PR, et al. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis and Rheumatism. 2011; 63: 2671-2680.
- Kodama T, Reddy P, Kishimoto C, Krieger M. Purification and characterization of a bovine acetyl low-density lipoprotein receptor. Proceedings of the National Academy of Sciences of the United States of America. 1988; 85: 9238-9242.
- Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type-1 macrophage scavenger receptor contains alpha-helical and collagenlike coiled coils. Nature. 1990; 343: 531-535.
- Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nature Medicine. 2002; 8: 1235-1242.
- Zhou HF, Chan HW, Wickline SA, Lanza GM. Pham CTN, alpha (v) beta (3)-Targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB Journal. 2009; 23: 2978-2985.
- Zhou HF, Hu G, Wickline SA, Lanza GM, Pham CT. Synergistic effect of antiangiogenic nanotherapy combined with methotrexate in the treatment of experimental inflammatory arthritis. Nanomedicine. 2010; 5: 1065-1074.
- Zhou HF, HYan H, Hu Y, Springer LE, Yang X, Wickline SA, et al. Fumagillin Prodrug Nanotherapy Suppresses Macrophage Inflammatory Response via Endothelial Nitric Oxide. ACS Nano. 2014; 8: 7305-7307.
- Lee SM, Kim HJ, Ha YJ, Park YN, Lee SK, Park YB, et al. Targeted chemo-photothermal treatments of rheumatoid arthritis using gold half-shell multifunctional nanoparticles. ACS Nano. 2013; 7: 50-57.
- Vallien G, Langley R, Jennings S, Specian R, Granger DN, Expression of endothelial cell adhesion molecules in neovascularized tissue. Microcirculation. 2000; 7: 249-258.
- Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer, Expert Opinion on Therapeutic Targets. 2007; 11: 1473-1491.
- Maehara A, Nishida K, Furutani M, Matsumoto E, Ohtsuka A, Ninomiya Y, et al. Light and electron microscopic detection of inflammation-targeting liposomes encapsulating high-density colloidal gold in arthritic mice. Inflammation Research. 2014; 63: 139-147.
- Banquy X, Leclair G, Rabanel JM, Argaw A, Bouchard JF, Hildgen P, et al. Selectins Ligand Decorated Drug Carriers for Activated Endothelial Cell Targeting. Bioconjugate Chemistry. 2008; 19: 2030-2039.
