
Case Report
Austin Anesthesiol. 2024; 4(1): 1010.
Anaphylactic Shock During a Percutaneous Treatment of Liver Hydatid Cyst: Case Report and Literature Review
Aarjouni Y*
Laboratory of Applied Organic Chemistry, Faculty of Sciences Semlalia, Universit’ Cadi Ayyad, Marrakech, Morocco
*Corresponding author: Aarjouni Y Laboratory of Applied Organic Chemistry, Faculty of Sciences Semlalia, BP2390, Universit’ Cadi Ayyad, Marrakech, Morocco. Email: yaarjouni@gmail.com
Received: May 20, 2024 Accepted: June 07, 2024 Published: June 14, 2024
Abstract
Percutaneous interventions have emerged as promising alternatives to surgery for the Hepatic hydatid cysts, caused by the larval stage of Echinococcus granulosus, pose substantial clinical challenges, particularly in endemic regions. While surgical excision has traditionally been the mainstay of treatment, percutaneous interventions offer less invasiveness and shorter recovery times. However, these minimally invasive procedures carry inherent risks, including the potential for rare but severe complications such as anaphylactic shock. This introduction sets the stage for a case report and literature review focusing on anaphylactic shock during percutaneous treatment of hepatic hydatid cysts, emphasizing the importance of recognizing and managing this complication for optimizing patient safety and outcomes.
Introduction
anaphylactic shock during percutaneous treatment of hepatic hydatid cysts is a rare but serious complication of Echinococcus granulosus infection. Hepatic hydatid cysts are prevalent in regions such as the Middle East, Mediterranean, and South America. The incidence of hepatic hydatid cysts varies widely, ranging from 1% to 25% of all cases of hydatid disease, depending on the geographic location and population studied [1]. Typically asymptomatic, hepatic hydatidosis may manifest as mild discomfort or pain in the abdominal region due to pressure on adjacent organs. Diagnosis often occurs incidentally during radiological investigations. However, the release of antigenic and highly toxic hydatid fluid from the cyst can lead to potentially fatal anaphylactic reactions, underscoring the need for vigilance and prompt management during percutaneous interventions for hepatic hydatid cysts.
We present a case of acute anaphylactic shock during a percutaneous procedure for a liver hydatid cyst in a young farmer patient.
Case Presentation
A 23-year-old female farmer presented with abdominal pain localized in the right hypochondruim which did not shift or radiate. The patient complained of malaise with nausea, vomiting, weight loss and intermittent fever within the last 6 months. The patient had no history of jaundice, cough or respiratory distress, her past medical history was unremarkable notably no allergic incidents. Physical examination found a Tenderness in the right hypochondrium with hepatomegaly. Abdominal computed tomography revealed an isolated hepatic cystic lesion measuring about 8,07 x 9,12 x 7 cm. There were no cysts in other abdominal viscera (Figure 1 & 2).
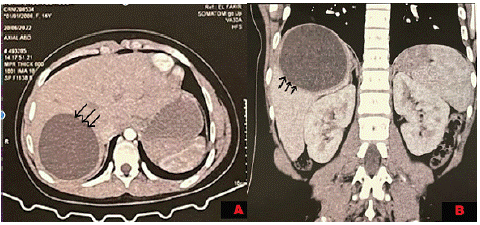
Figure 1: Image showing the hydatid cyst. A: axial section and B: coronal section.
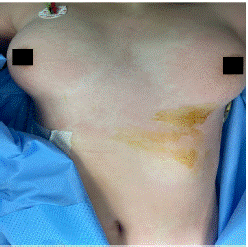
Figure 2: Cutaneous sign in the chest
The patient was scheduled for percutaneous treatment, including puncture and aspiration of cyst contents, injection of scolicidal agents, with re-aspiration (PAIR).
At the pre-anaesthetic consultation, there was no notable medical history and no alcohol or smoking habits, No personnel or familial history of allergy was documented. The clinical examination revealed a weight of 75kg, a height of 1.70m and a body mass index of 26 kg/m, good exercise tolerance with a functional capacity of over 4 METs. Blood pressure was 120/65, with a pulse rate of 83 beats/min. Preoperative examinations of the cardiovascular and respiratory systems were normal. Electrocardiography (ECG) and chest X-ray were unremarkable. Upper airway assessment showed native teeth, a thyro-chin distance greater than 6.5 cm, a Mallampati score of II and a mouth opening greater than 35 mm with a soft neck.
At the end of this evaluation, the patient was classified as ASA I, Mallampati II, and scheduled for surgery at low hemorrhagic and cardiovascular risk : percutaneous treatment of liver hydatid cyst.
The patient admitted to the operating room, placed in the supine position a standard continuous monitoring including Heart Rate (HR), arterial oxygen saturation (SpO2), and Non-invasive Pression (NIP) was installed. After the installation of a nasal cannula with an oxygen flow of 5l and a catheterization of a peripheral vein, an IV 2 g of cefazolin was administered without. problems notably; The initial parameters were correct the (HR) at 78 beats/min, NIP at 124/76 mmHg, and SpO2 at 99%, the procedure was performed using local anesthesia coupled with mild intravenous sedation, anesthesia was introduced by fentanyl (50ug) and titration of propofol. The procedure was performed under Ultrasound (US) guidance, To prevent peritoneal dissemination, the puncture site was chosen from an area that included a pass through normal liver tissue. Local anesthesia using 2% lidocaine was given under sterile conditions. The cyst was punctured using an 18-gauge Chiba needle (Cook Medical Bloomington, IN, USA) with US guidance. More than half of the estimated cyst volume was aspirated. The aspirated specimens were submitted for cytological and biochemical assessment. After initial fluid aspiration, the endocyst detached, which is highly suggestive of a hydatid cyst. Then, 30% hypertonic saline is injected equivalent to an estimated one-third of the cyst volume into the cyst cavity. After, all fluid in the cavity was re-aspirated. During the re-aspiration of the fluid the patient had presented hypotension (NIP: 65/34 mmHg), tachycardia (HR: 110 beats/min). The manipulations were stopped. Pulmonary auscultation was normal; in particular there was no wheeze. Monitoring did not notice any changes in ECG and no macroscopic evidence of infection. was noted by surgeon. cutaneous signs were noted on the face, the arm and neck (Figure 3 & 4). Blood loss was low (approximately ≈150 mL). Diagnosis of anaphylactic shock was highly suspected. A second peripheral venous catheter (16 gauge), and the patient was intubated to prevent angioedema, arterial catheter were placed. Fluid resuscitation (saline 0.9%) 500 mL per 500 mL (with a total of 1500 mL) and IV boluses of ephedrine (30 mg total) allowed only a slight improvement (NIP: 69/43 mmHg). Epinephrine boluses (100 μg to a total of 300 μg) were administered and relayed by continuous infusion (0.08 μg/ kg/min) via a central venous catheter. This therapeutic has stabilized the hemodynamic status (NIP: 109/56 mmHg and HR: 90 beats/min) and so continue the procedure. A bolus of 200 mg of hydrocortisone was administered. No need for additional bolus of epinephrine or an increase in infusion rate were required. After removing of surgical draping, presence of skin signs all over the body has been noticed (Figures 5 & 6). Serum tryptase was not measured because of its nonavailability in our hospital. The patient was transferred to ICU. Postoperative course was unremarkable with extubation 1 h later and a withdrawal of drugs 3 h later. The patient was discharged home after 5 days of hospitalization.
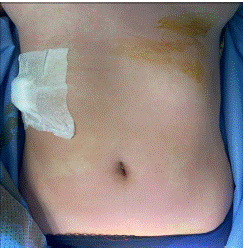
Figure 3: Cutaneous sign in the chest.
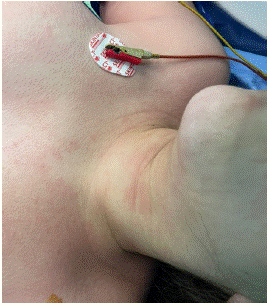
Figure 4: Cutaneous sign in the neck.
Discussion
Allergic reactions and anaphylaxis are IgE-mediated immediate hypersensitivity reactions that occur when antigen-specific IgE, bound to receptors on mast cells and basophils, are cross linked by the antigen, activating the cells to rapidly release a variety of mediators such as histamine, enzymes and lipid mediators [2].
While anaphylactic reactions and allergic symptoms are usually observed in cases of treatment-related rupture of echinococcal cysts, they may also occur spontaneously. The symptoms vary from mild urticaria to anaphylactic shock [3]. The presence of specific IgE in serum of patients is a well-known feature of CE with levels varying according to cyst number, location, morphology, disease severity, and cyst stage [4,5].
Although 75% of patients have detectable levels of specific IgE, antigens can be detected in 100% of patients. However, the predictive value of IgE for developing a reaction has not yet been studied [6]. Echinococcus allergens were studied with the intention of improving the performance of diagnostic tests. Three proteins were identified (EgEF-1b/d, EA21 and Eg2HSP70), by screening an E. granulosus cDNA library with IgE antibodies from patients with and without allergic skin manifestations, showing IgE-binding reactivity significantly different from one group to another [7-9]. However, the identification of such reactivity by a patient's IgE as a predictive factor for the development of anaphylaxis has never been studied. Another interesting, but as yet unexplored, hypothesis is the use of these allergens as part of desensitization therapy [10], the practicality of preoperative treatment for the prevention of anaphylactic reactions having never been demonstrated.
The pathophysiology of anaphylactic reactions in EC is still unclear, but is most commonly explained by rupture of the cyst wall integrity with spillage and translocation of the cyst's allergenic contents into the host circulation. Despite this, echinococcal cyst rupture does not always or necessarily lead to anaphylactic reactions.
In the literature, five fatal cases were reported out of 5943 PT procedures performed. These cases occurred during the treatment of 5517 echinococcal cysts, giving an overall mortality rate of 0.08% (5 out of 5943) and 0.09% (5 out of 5517) respectively. The overall mortality rate due to fatal anaphylaxis was 0.03% (2 of 5943) and 0.04% (2 of 5517) respectively (Table 1) [11,12].
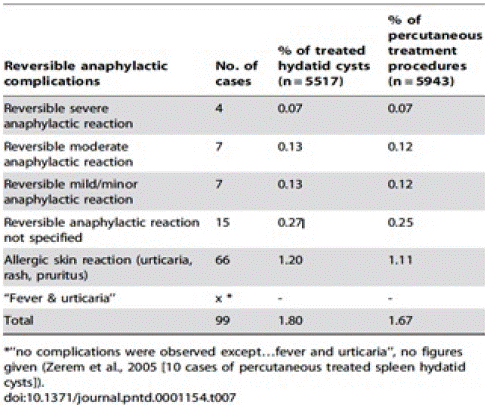
Table 1: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
The reversible complications fall into three categories: anaphylactic, potentially anaphylactic, and non-anaphylactic: In total, 99 reversible anaphylactic reactions were reported in 5943 PT procedures for the treatment of 5517 echinococcal cysts. Therefore, reversible allergic reactions complicated 1.7% of all PT procedures, corresponding to 1.8% of all treated echinococcal cysts (Table 2) [13].
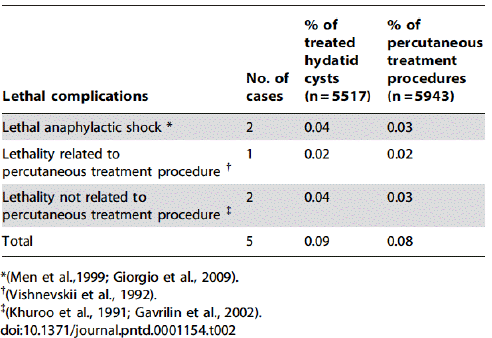
Table 2: Overall reversible anaphylactic reactions due to percutaneous treatment of hydatid cysts.
The retrospective evaluation of publications on PT related complications is certainly limited by a number of factors such as non-uniform definitions of anaphylactic events, the merging of data from different kind of studies – covering different PT methods in different settings and dealing with a different composition of clinical cases – and the denominator issue. Due to the retrospective
nature of our review and because we can only analyze published data, a publication bias can also be at work. It can be argued that severe events (e.g., severe anaphylaxis) might be more likely be published. But one could counter that event assumed to be common (especially the often-quoted PT related anaphylaxis) might not as readily be published. Nevertheless, we consider the analysis of the existing published literature a justified approach as no other source of more accurate data is currently available.
Future work in this area is needed to investigate the pathophysiology of anaphylactic reactions in CE and to prospectively study the potential relationship between clinical variables such as location, number, size, stage of the cyst, and risk of anaphylactic reactions.
In our cases, anaphylactic shock was attributed to hydatid fluid due to the elimination of other causes and occurred at a distance of induction (time of injection of drugs). Nevertheless, the diagnosis of anaphylaxis should be established by various immunologic immunofluorescence reactions and immuno-electrophoresis hemagglutination [14]. In our center, these reactions were unavailable and not realized in our patient.
The treatment of anaphylactic shock during surgery is facilitated by the prior installation of monitoring, of vascular access and airway access if general anesthesia. This treatment consists of stopping administration of any medication, stopping momentary intervention, massive fluid resuscitation, and administration of vasopressor and corticosteroids. Fluid replacement should be assured by crystalloids. For vasopressor, epinephrine is the first-line treatment in most guidelines on perioperative management of anaphylaxis [15,16]. Glucocorticoids are often administered in acute phase of anaphylactic shock, although their effects are delayed several hours; a beneficial role has been suggested to prevent the recurrence of manifestations of anaphylaxis in the late phase [17]. Our patient has received a saline solution, corticosteroids, and epinephrine by bolus and infusion. Hemodynamic response was good and authorized to continue the procedure.
the prevention of anaphylactic reaction is mainly related to the technique used, whether percutaneous or surgical. While surgery legitimately maintains a central role in complicated cysts (rupture, biliary fistulas, compression of vital structures, bacterial superinfection, haemorrhage), cysts at high risk of rupture, or large cysts with many daughter vesicles, that are not suitable for percutaneous treatment approaches, PT has shown to be a safe and effective alternative for many patients with suitable cysts. What is needed now are evidence-based criteria to allocate the patient to the most appropriate treatment option according to the specific situation [13].
References
- https://www.who.int/fr/news-room/fact-sheets/detail/echinococcosis
- AK Abbas, AH Lichtman, S Pillai. Cellular and molecular immunology. Elsevier Brasil. 2007.
- DA Vuitton. Echinococcosis and allergy. Clin Rev Allergy Immunol. 2004; 26: 93–104.
- Rigano R, Loppolo S, Ortona E, Margutti P, Profumo E, Ali MD, et al. Long-term serological evaluation of patients with cystic echinococcosis treated with benzimidazole carbamates Clin Exp Immunol. 2002l 129: 485–492.
- J Torcal, Navarro-Zorraquino M, Lozano R, Larrad L, Salinas JC, et al. Immune response and in vivo production of cytokines in patients with liver hydatidosis. Clin Exp Immunol. 1996; 106: 317–322.
- A Aceti, Celestino D, Teggi A, Caferro M, Pennica A, Grilli A, et al. Histamine release test in the diagnosis of human hydatidosis. Clin Exp Allergy. 1989; 19: 335–339.
- E Ortona, Margutti P, Delunardo F, Vaccari S, Rigano R, Profumo E, et al. Molecular and immunological characterization of the C-terminal region of a new Echinococcus granulosus Heat Shock Protein 70. Parasite Immunol. 2003; 25: 119–126.
- E Ortona, Margutti P, Vaccari S, Rigano R, Profumo E, Buttari B, et al. Elongation factor 1 β/d of Echinococcus granulosus and allergic manifestations in human cystic echinococcosis. Clin Exp Immunol. 2001; 125: 110–116.
- E Ortona, Vaccari S, Margutti P, Delunardo F, Rigano R, Profumo E, et al. Immunological characterization of Echinococcus granulosus cyclophilin, an allergen reactive with IgE and IgG4 from patients with cystic echinococcosis. Clin Exp Immunol. 2002; 128: 124–130.
- JR Kambam, R Dymond, M Krestow, RE Handte. Efficacy of histamine H1 and H2 receptor blockers in the anesthetic management during operation for hydatid cysts of liver and lungs. South Med J. 1988; 81: 1013–1015.
- AV Gavrilin, GI Kuntsevich, Vishnevskiui. Ultrasound-assisted puncture method of treatment of hepatic hydatid cysts.
- MS Khuroo, SA Zargar, R Mahajan. Echinococcus granulosus cysts in the liver: management with percutaneous drainage. Radiology. 1991; 180: 141–145.
- A Neumayr, Troia G, de Bernardis C, Tamarozzi F, Goblirsch S, Piccoli L, et al. Justified concern or exaggerated fear: the risk of anaphylaxis in percutaneous treatment of cystic echinococcosis—a systematic literature review. PLoS Negl. Trop Dis. 2011; 5: e1154.
- F Estelle, R Simons, BY Thong, R Ardusso, MB Bilo, YM El-Gamal. World Allergy Organization anaphylaxis guidelines: Summary: Food allergy. J Allergy Clin. Immunol. 2011; 127: 587–593.
- M Kroigaard, Garvey LH, Gillberg L, Johansson SGO, Mosbech H, Florvaag E, et al. Scandinavian Clinical Practice Guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand. 2007; 51: 655–670.
- D Longrois, C Lejus, I Constant, M Bruyère, PM Mertes. Treatment of hypersensitivity reactions and anaphylactic shock occurring during anaesthesia. Annales Francaises D’anesthesie et de Reanimation. 2011; 30: 312–322.
- W Kloeck, Cummins RO, Chamberlain D, Bossaert L, Callanan V, Carli P, et al. Special resuscitation situations: an advisory statement from the International Liaison Committee on Resuscitation. Circulation. 1997; 95: 2196–2210.