Abstract
Introduction: Patients for Coronary Artery Bypass Grafting (CABG) are often treated with antiplatelet medications, most frequently Aspirin (ASA) and Adenosine-Diphosphate (ADP) -receptor antagonists. The drugs seem effective in reducing thromboembolic events when awaiting surgery, but carry a risk of increased bleeding and complications. Although inconsistent, the latest recommendation is to continue ASA, but discontinue ADP 5-days before surgery. The aim of this study was to evaluate possible associations between continued antiplatelet treatments and postoperative thromboembolic events.
Methods: A propensity based analysis on data from our mandatory Western Denmark Heart Registry (2007-2014). Eligible procedures were on/off-pump CABG with/without valve replacement (N=10.608). The endpoints were mortality and/or new ischaemic event (CAG/PCI/CABG) within 6 months together with transfusions and in-hospital incidence of stroke, MI and need for dialysis.
Results: Patients continuing ASA had lower risk of postoperative new CAG in crude analysis, but otherwise no differences in outcomes between ASA and controls in crude or adjusted analysis. Postoperative drainage was marginally higher after ASA, but without difference in re-exploration due to bleeding. Comparing Clopidogrel and ASA, the only difference was a lower postoperative risk of stroke in the clopidogrel patients after adjustment. The Clopidogrel patients received more blood-products, bled more and were more often reexplored due to bleeding.
Conclusion: Patients continuing ASA carry a minor risk of increased postoperative bleeding. The risk is increased if clopidogrel is not discontinued before surgery. The continued therapy had no effect on the frequency of postoperative thromboembolic events. The optimal timing of antiplatelet therapy still needs solid evidence.
Keywords: Coronary artery bypass grafting; Antiplatelet treatment; Ischemic events; Postoperative bleeding; Re-exploration
Glossary of Abbreviations
ACS: Acute Coronary Syndrome; ACT: Activated Clotting Time; ADP: Adenosine Diphosphate; AP: Antiplatelet; ASA: Acetylsalicylic Acid; AVR: Aortic Valve Replacement; CABG: Coronary Artery Bypass Grafting; CAG: Coronary Angiography; CPB: Cardiopulmonary Bypass; COLD: Chronic Obstructive Lung Disease; MI: Myocardial Infarction; MVR: Mitral Valve Replacement/ Repair; OR: Odds Ratio; PCI: Percutaneous Coronary Intervention; WDHR: Western Denmark Heart Registry
Introduction
Patients with ischemic heart disease referred to cardiac surgery are often treated with Antiplatelet (AP) agents like Acetyl Salicylic Acid (ASA), oral Adenosine Diphosphate (ADP-) receptor antagonists and glycoprotein IIb/IIIa receptor inhibitors, but also different types of anticoagulation like Fondaparinux and Dalteparin. The longterm standard of care is the irreversible platelet inhibitor, ASA. The combined or stand- alone treatment with oral ADP-receptor antagonists is recommended for patients with actual or recent Acute Coronary Syndrome (ACS) or Percutaneous Coronary Intervention (PCI) [1-2]. Clopidogrel inhibits platelet activation and thereby aggregation via the ADP-receptor on the platelet membrane.
The only way platelet function can be restored is either by transfusion or by de novo synthesis of platelets. A platelet lifespan is 8-10 days and thus after 3-5 days half the platelets are renewed which is considered sufficient to normalize bleeding time [3]. The uses of the different agents at the time of surgery carry both benefits and risks. The drugs seem effective in reducing thromboembolic events in patients awaiting surgery [4-5], but also carry the risk of increased per- and postoperative bleeding [5-6]. Thus optimal timing for discontinuation of antiplatelet therapy prior to surgery is important. Until recently the international guidelines recommended discontinuing ASA two to ten days before elective cardiac surgery, while they advised that the ADPreceptor antagonists should be withheld for at least five days before elective Coronary Artery Bypass Grafting (CABG) [7-8]. The latest recommendations are to continue ASA [8] but still discontinue ADPreceptor antagonists five days prior to surgery [8-10].
However, the most recent results regarding discontinuation of ASA are still conflicting [11-12]. The aim of the present study was to evaluate if continued AP treatment in connection with cardiac surgery has impact on postoperative thromboembolic complications, with the hypothesis being that this group of patients have an increased risk of postoperative complications including new ischemic events and mortality.
Patients and Methods
An analysis was carried out with data from three cardiac centers registered in the Western Denmark Heart Registry (WDHR) [13] from 2007-2014. The health care in Denmark is fully tax-funded for all Danish residents. Theinvolved centers provide cardiac surgery for a mixed rural-urban population constituting approximately fifty-five percent of the entire Danish population.
Registration in WDHR is mandatory and completed perioperative by the surgeon and attending anesthetist and includes detailed patient-, surgery-, anesthesia-, intensive care related data together with in-hospital complications. Data quality is ensured using automatic validation rules at data entry combined with systematic validation procedures and random spot-checks of data after entry. The database obtains daily mortality information from the Danish Civil Registration system [14], which keeps record on residency, migration, civil- and vital status of all Danish citizens including date of death.
Eligible procedures were CABG, alone or combined with Aortic Valve Replacement (AVR), Mitral Valve Replacement/Repair (MVR) and MAZE procedures for atrial fibrillation or combination, with maximum triple procedures included. Both on-pump and off-pump CABG procedures were included regardless of urgency status. Exclusions were 150 patients scheduled for aortic surgery, 373 patients with invalid civil registration number or missing required information together with 320 patients receiving another treatment than ASA and/or Clopidogrel like Fondaparinux, leaving 10,608 patients in the study cohort (Figure 1). The study was approved by the Danish Data Protection Agency (record number 1-16-02-541- 16). According to Danish law use of data from Danish registers for research does not require ethical approval.
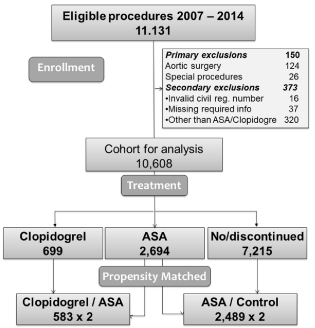
Figure 1: Cohort 2006 to 2014 of patients undergoing coronary artery bypass
grafting. Overall numbers, exclusions, groups before and after propensity
score match.
Perioperative procedure
During the first part of the study period, it was standard care for patients to discontinue ASA and oral ADP-receptor antagonist five days prior to surgery. The surgeon evaluated indication for continued treatment and if indicated, patients with increased risk of ischemic events continued aspirin until the day of surgery. The procedure was adjusted during 2013 and from that time point ASA was continued until the day before surgery. ADP-receptor antagonists were usually discontinued five days prior to surgery but a number of patients continued treatment until the day before surgery. Vitamin K-antagonists were usually withheld two to three days prior to surgery and the effect controlled by laboratory values and antidotes given if necessary.
The surgical techniques were at the discretion of the surgical team. Patients received general anesthesia with invasive hemodynamic monitoring and standardized Cardio Pulmonary Bypass (CPB). Myocardial protection was achieved by either intermittent cold crystalloid or blood cardioplegia. Patients were maintained either normothermic or mildly hypothermic. CPB was established using a closed system consisting of tubing, an arterial filter with heparin coating, a hollow fiber-membrane oxygenator, and a venous cardiotomy reservoir. Heparin was administered to achieve an Activated Clotting Time (ACT) above 400 seconds and was neutralized after CPB using protamine-sulphate. During CPB the blood flow was kept at 2.4/L/min/m2 and the mean arterial blood pressure at 50-70 mmHg. Residual blood from the CPB circuit was routinely re-transfused at the end of surgery.
Blood products were given at the discretion of the attending anesthesiologist or surgeon, based on local transfusion guidelines and the national recommendations for blood transfusion [15]. The majority of patients received antifibrinolytic treatment in the form of tranexamic acid, while a minor fraction of patients received aprotinin.
Postoperatively ASA was as standard resumed on the first postoperative day. Clopidogrel was resumed individually and decided by the surgeon.
Patient and outcome characteristics
Primary endpoints were 6-months mortality (all deaths occurring within six months of the primary surgery) and/or new possible ischemic event, defined as re-interventions in the form of Coronary AngioGraphy (CAG) / PCI / CABG within 6 months post-surgery. Only CAG with the indication “suspected myocardial infarction”, “unstable or stable angina pectoris”, and “complication after CABG” or “control after CABG” were included. CAG with other indications, e.g. “cardiac arrhythmia”, planned “Completion PCI” were excluded together with planned hybrid procedure (patients preoperatively scheduled for combined CABG/PCI).
Secondary outcome parameters were 30-days mortality and the postoperative incidence of new Myocardial Infarction (MI), stroke or new need for dialysis during the index hospitalization. MI was defined by the following criteria: the occurrence of a new Q-wave and/or a CK-MB more than five times the upper reference level. Stroke was a combined outcome index defined as both transitory ischemic attacks lasting less than 24 hours and/or a new neurological deficit lasting more than 24 hours. We did not distinguish between hemorrhagic and ischemic stroke. We registered postoperative acute renal failure as new need for dialysis without differentiating between the actual mode of renal replacement therapy or the precise indication.
Tertiary outcomes were transfusions and re-exploration due to bleeding.
Statistical analysis
We used propensity score matching to reduce the risk of bias due to confounding and the non-random discontinuation of preoperative anti-platelet medication. Two independent propensity matched cohorts were established; 1 (continued stand-alone ASA treatment versus discontinued treatment; 2) continued Clopidogrel (alone or combination with Clopidogrel and ASA) treatment versus standalone ASA treatment.
The included covariates in the propensity match were; age, sex, Chronic Obstructive Lung Disease (COLD), peripheral artery disease, poor mobility/preoperative central nervous disease, s-creatinine > 200 μmol/l, active endocarditis, previous cardiac surgery, critical preoperative state, unstable angina, myocardial infarction within 90 days, left ventricular function (ejection fraction > 50/30-50/<30), acute surgery and type of surgery (Standalone CABG/double procedure/ triple procedure), all above EuroSCORE I/II criteria. Further, CPB time (Off pump/≤ 120 minutes/> 120 minutes), insulin dependent diabetes, preoperative Dalteparin, perioperative vasoconstrictors, perioperative inotropes, contrast +/- 7 days of surgery, body mass index (BMI<25.0/25-29.9/30-39.9/≥40.0).
Patients receiving ASA were matched with a control with no or discontinued ASA with the nearest propensity score within a maximum caliper range of ± 0.025 and without replacement. We were able to match 2489 of the 2694 (92.4%) patients continuing ASA treatment. The second analysis was patients continuing Clopidogrel (+/- ASA) matched with patients continuing standalone ASA treatment, where 583 of 699 (83.4%) of these patients were matched. Standardized differences of all matching criterions before and after propensity scores are shown in (Figure 2) and actual numbers in (Table 1). Patients receiving Dalteparin were included in the match and the analysis (Clopidogrel (+/-ASA)/ASA n=184/182 and ASA/ control n= 285/295).
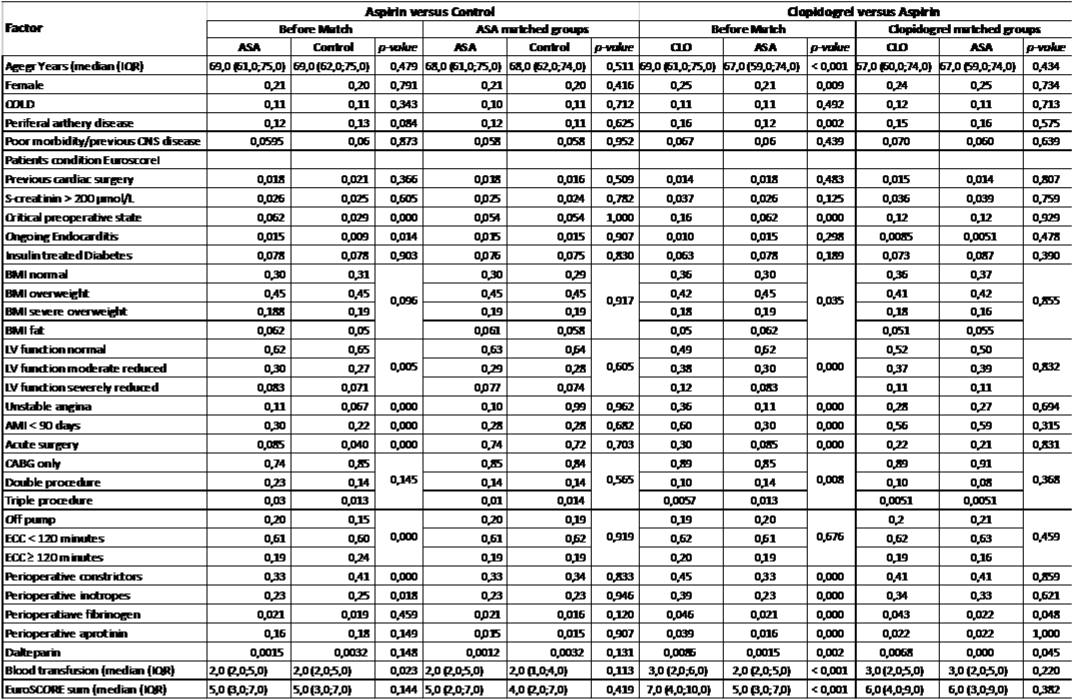
Table 1: Distribution of factors before and after the propensity score matching. COLD=Chronic Obstructive Lung Disease, BMI=Body Mass Index; LV=Left
Ventricular; AMI=Acute Myocardial Infarction; CABG=Coronary Artery Bypass Grafting; ECC=Extra Corporal Circulation Time. Statistics: *) χ2-test; !) Mann-
Whitney test.
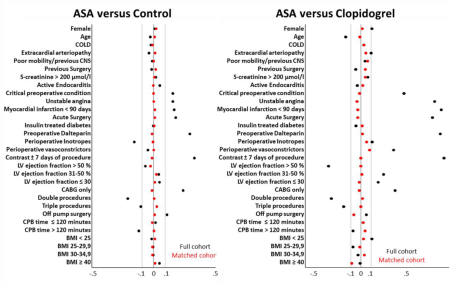
Figure 2: Standardized differences of all matching criterions before and after propensity score. The included covariates were; age, sex, COLD (chronic obstructive
lung disease), peripheral artery disease, poor mobility (preoperative central nervous disease, EuroSCORE 1), s-creatinine > 200 μmol/l, previous cardiac surgery,
endocarditis, critical preoperative state/condition, unstable angina, myocardial infarction ≤ 90 days before surgery, left ventricular function (3 groups), acute
surgery, procedure type (3 groups), all above EuroSCORE 1 and 2 criteria. Extra corporal circulation time (3 groups), insulin dependent diabetes, preoperative
dalteparin, perioperative vasoconstrictors, perioperative inotropes, contrast +/- 7 days of procedure, BMI group (4 groups).
We used conditional logistic regression to take into account the non-independency within each pair when estimating odds ratio (OR) for the specified outcomes. Both crude and adjusted analyzes wre carried out. The adjustment factors were blood transfusion (longitudinal 0-8 units), year before/after 2013, department and number of grafts in CABG.Time related factors were furthermore analyzed as cumulated risk in a Kaplan Meier plot. Distribution of factors before and after the propensity score matching – when indicated χ2-test or Mann-Whitney test. P-values less than 0.05 were considered statistically significant.
Propensity scores and conditional regression analysis were performed using the statistical software package Stata® 13.0 package (StataCorp LP, Texas, US). All other analyses were performed with MedCalc Statistical Software version 15.8 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2015).
Results
In crude analysis patients continuing ASA had lower risk of postoperative CAG in crude regression analyzed. However, the differences did not remain after adjustment analyses (Table 2). There was no difference in 30-days or 6-months mortality comparing patients continuing ASA with controls, and the groups were also without differences in the other outcomes: stroke, dialysis, AMI and PCI/CABG within 6 months both in crude and adjusted for potential confounders (Table 2, upper panel).
Outcome factor
Crude analysis
Adjusted analysis
ASA versus Control
Mortality 30-days
0.93 (0.65 - 1.34)
0.98 (0.55 - 1.77)
Mortality 6-month
1.05 (0.79 - 1.41)
1.32 (0.87 - 2.03)
Stroke
0.88 (0.58 - 1.32)
1.09 (0.64 - 1.87)
New Dialysis
0.75 (0.52 - 1.09)
1.07 (0.51 - 2.24)
AMI
0.93 (0.73 - 1.21)
1.04 (0.74 - 1.48)
CAG < 6-month
0.72 (0.58 - 0.89)
0.85 (0.63 -1.14)
PCI/CABG < 6-month
0.82 (0.58 - 1.14)
0.81 (0.51 - 1.29)
Clopidogrel versus ASA
Mortality 30-days
1.20 (0.71 - 2.04)
1.19 (0.60 - 2.40)
Mortality 6-month
1.07 (0.70 - 1.66)
1.09 (0.61 - 1.93)
Stroke
0.52 (0.24 - 1.13)
0.24 (0.07 - 0.88)
New Dialysis
0.80 (0.54 - 1.34)
0.44 (0.12 - 1.52)
AMI
0.85 (0.54 - 1.34)
0.85 (0.52 - 1.40)
CAG < 6-month
1.05 (0.96 - 2.71)
0.95 (0.62 - 1.48)
PCI/CABG < 6-month
1.57 (0.93 - 2.64)
1.49 (0.68 - 3.19)
Table 2: Crude and adjusted odds-ratio and 95% confidence limits of selected outcome parameters in patients divided on ASA and control (Upper panel) and patients divided on Clopidogrel and ASA (lower panel). Adjustment factors blood transfusion (longitudinal 0-8 units), year before/after 2013, department and number of grafts in CABG. Stroke, New dialysis and AMI are in-hospital complication.
Comparing the ASA group with Clopidogrel (+/- ASA) patients there was no difference in 30-days or 6-months mortality. There was a lower risk of stroke in the Clopidogrel-treated group after adjustment. Otherwise the groups did not differ in other parameters (Table 2, lower panel). Evaluation of the individual factors in adjusted analysis showed that fewer anastomoses increased the risk of a new ischaemic event within the first 6 months after primary surgery (Table 3). The adjusted conditional regression revealed that blood transfusion was an individual risk factor in almost all outcomes (Table 3). Year of procedure did not show independent impact and analyzing number of ischemic events within 6 month in patients continuing on ASA did not show any difference from 2007 to 2014 (P=0.204; χ2-test). Postoperative bleeding and platelet transfusions were marginally higher after ASA, but without difference in re-exploration due to bleeding. Patients in the Clopidogrel group bled more and were more often re-operated due to bleeding in addition to a higher transfusion rate (with all types of blood products), (Table 4). The Kaplan-Meier plot showed a significant difference between ASA and control patients. The plot shows that the increased risk of ischemic events is primarily during the first days after which the curves are parallel (Figure 3, upper panel). No difference was found between Clopidogrel (+/- ASA) and ASA patients (Figure 3, lower panel).
CAG
PCI/CABG
Event < 6 month
ASA
0.85 (0.63 - 1.14)
0.81 (0.51 - 1.29)
0.82 (0.62 - 1.08)
Year group
1.24 (0.84 - 1.83)
0.66 (0.38 - 1.17)
0.93 (0.66 - 1.33)
No of anastomoses
0.88 (0.76 - 1.03)
0.81 (0.64 - 1.03)
0.83 (0.72 - 0.97)
Blood
1.14 (1.05 - 1.24)
1.19 (1.02 - 1.35)
1.16 (1.07 - 1.26)
Clopidogrel
0.95 (0.62 - 1.48)
1.48 (0.70 - 3.19)
1.19 (0.78 - 1.82)
Year group
1.28 (0.55 - 2.99)
0.63 (0.17 - 2.23)
0.81 (0.37 - 1.78)
No of anastomoses
0.89 (0.68 - 1.16)
0.63 (0.42 - 0.95)
0.82 (0.63 - 1.06)
Blood
1.10 (0.99 - 1.22)
1.16 (0.98 - 1.36)
1.12 (1.01 - 1.22)
Table 3: Individual odds-ratios (95 CL) of factors in adjusted conditional regression analysis of ASA versus control (upper panel) and Clopidogrel versus ASA (lower panel). Year group is before/after 2013, No of anastomoses is risk per grafts (longitudinal) and blood transfusion is risk per transfusion (longitudinal).
Parameter
ASA / Control
ASA / Clopidogrel
Control
ASA
ASA
Clopidogrel
Transfusions
No platelets
84.9 %
78.9 %
<0.0001
73.9 %
53.3 %
<0.0001
1-2 units
9.9 %
15.8 %
17.8 %
28.6 %
3-4 units
3.2 %
3.7 %
5.5 %
11.5 %
5-6 units
1.4 %
1.0 %
1.4 %
3.9 %
> 6 units
0.6 %
0.5 %
1.4 %
2.6 %
No plasma
86.1 %
84.0 %
0.054
79.6 %
63.5 %
<0.0001
1-2 units
7.3 %
7.2 %
7.4 %
16.1 %
3-4 units
3.4 %
4.6 %
6.2 %
10.8 %
5-6 units
1.2 %
1.8 %
3.1 %
4.1 %
> 6 units
2.0 %
2.5 %
3.8 %
5.5 %
No blood
73.5 %
74.2 %
0.709
64.3 %
51.8 %
0.0004
1-2 units
14.7 %
13.3 %
17.7 %
21.3 %
3-4 units
5.8 %
5.7 %
7.0 %
11.8 %
5-6 units
2.2 %
3.0 %
4.6 %
6.3 %
> 6 units
3.7 %
3.9 %
6.3 %
8.7 %
Re-do bleeding
3.9 %
4.5 %
0.135
4.5 %
9.6 %
0.0006
Drainage
0-400 ml
34.6 %
28.9 %
0.0003
28.1 %
21.9 %
0.0001
401-800 ml
37.1 %
39.7 %
38.4 %
30.8 %
801-1200 ml
14.2 %
16.6 %
16.7 %
19.7 %
> 1200 ml
14.1 %
14.9 %
16.8 %
27.7 %
Table 4: The impact on bleeding, transfusion and re-do due to bleeding divided on treatment group in the two propensity matched cohorts.
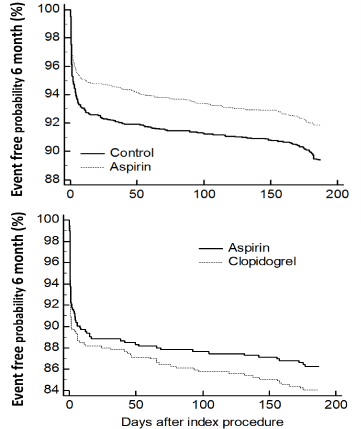
Figure 3: Overall event-free probability divided on aspirin/control. Hazard
ratio 0.76 (0.63-0.91); P=0.029 (Upper panel) and overall event-free
probability divided on clopidogrel/aspirin. Hazard ratio 1.17 (0.87-1.58);
P=0.31, (Lower panel).
Discussion
Previous studies investigating antiplatelet therapy have primarily focused on the risk of bleeding. The present study supports the evidence of this risk but additionally demonstrates no increased risk of thromboembolic events for patients discontinuing their ASA or Clopidogrel a few days prior to cardiac surgery. Several studies support a protective effect of continuing ASA [16-17]. Jacob et al. [3] investigated early (6 or more days) versus late (within five days of surgery) discontinuation of ASA and found no difference in postoperative MI or stroke or in-hospital all-cause mortality. Gielen et al. [18] made a subgroup analysis on 1065 patients from the FIBER study. They tried to identify the optimal day for discontinuing antithrombotic treatment before CABG. This was, however, not possible and they found no clinical relevant effect on blood loss, major adverse cardiovascular and cerebrovascular events. Interestingly, last use two days before surgery or earlier resulted in a 30% reduction (p<0,001) of patients receiving platelet transfusions compared with continuation of dual antiplatelet medication until the day before surgery. The ATACAS study investigated whether ASA could reduce the occurrence of death and thrombotic complications in at-risk patients who were undergoing coronary artery surgery [12]. They found no association between preoperative use of aspirin, a decreased risk of death or thrombotic complications nor a higher risk of surgical bleeding, need for transfusion or need for reoperation. Our study supports these findings as we saw no increased mortality and no increased risk in other outcomes, dialysis or AMI.
In our data Clopidogrel treatment did not affect the primary endpoints, which was also the conclusion by Kulik et al. [19]. We found a decreased risk of stroke in the Clopidogrel group. An explanation might be that the risk of stroke is a perioperative phenomenon with Clopidogrel still having a protective effect. A recently published study shows no additional reduction in ischemic events with dual antiplatelet therapy one year after CABG compared to ASA alone [20]. Others have suggested that its continuation up to the date of surgery may increase perioperative morbidity and mortality [21-22]. It has been reported that the mortality risk is greatest when the drug is given within 48 hours of surgery [22].
There is an increased risk of bleeding when continuing both ASA and Clopidogrel. This is well known [3,21,23] and the risk should be evaluated against the risk of thromboembolic complications when discontinuing antiplatelet therapy. Increased bleeding and transfusion of allogeneic blood products are indirect risk factors for increased mortality and risk of later ischemic events [24-25]. Discontinuing Clopidogrel for a few days is generally recommended to reduce bleeding and transfusion requirements especially in highrisk- patients. According to The ESC Guidelines for the management of STEMI, acute surgery should not be delayed due to Clopidogrel treatment [10]. This is supported by a meta-analysis of 34 studies [23], arguing that the risk of reoperation due to Clopidogrel has decreased through the last decade and no longer differs from controls. Further they found no difference in the triple end point of death, MI or stroke whether Clopidogrel was continued until surgery or not. Discontinuation of Clopidogrel 5-7 days before operation did not confer increased risk of worse cardiac outcomes. Firanescu et al. [26] found that the blood loss and the use of blood products in a group ceasing Clopidogrel three days preoperatively were similar to that of the group ceasing treatment five days preoperatively.
Responsiveness to Clopidogrel and ASA is not uniform in all patients and is subject to inter- and intra-individual variability [27]. Platelet function testing is recommended as a guide to antiplatelet therapy interruption rather than arbitrary use of a specified period of delay in patients undergoing CABG surgery [8,28]. Patients treated with ASA and Clopidogrel less than 5 days before CABG who have a preoperative ADP-induced platelet aggregation ≥50% have a bleeding risk similar to those receiving aspirin as mono-therapy. Reduced platelet reactivity to ADP can predict postoperative bleeding in CABG patients on dual antiplatelet therapy [29]. On the contrary, no randomized controlled trial has demonstrated that routine platelet function testing or genetic testing to guide ADP-receptor antagonist therapy improves outcome; thus, the routine use of platelet function and genetic testing is not recommended in general [8,28].
The postoperative standard in this study was that ASA was resumed on the first postoperative day and Clopidogrel restarted on an individually evaluation by the surgeon. Several studies, including meta-analyses and consensus guidelines confirms that early postoperative aspirin improves vein graft patency, reduces ischemic complications and improves survival in patients undergoing CABG [8,19,28]. Regarding Clopidogrel AHA/ACC 2016 guidelines [30] recommend that Clopidogrel is resumed postoperatively but do not elaborate appropriate timing. Former studies recommend that Clopidogrel should be given after CABG-surgery as soon as considered safe [8,28,31].
It has previously been suggested to avoid CPB to minimize the risk of bleeding [32]. As the CPB time is a used as a factor in the propensity model we are not able to verify this. However, our results indicate in a standard regression analysis of the entire cohort that a short CPBtime (<120 min) decreases the risk of a new ischemic event within the first 6 months, but the cause is outside the scope of this article and remains unclear. Patients with longer CPB-time are expected to have more complex disease and may be more atherosclerotic. Furthermore a longer CPB-time may increase the inflammatory reaction, as well as the activating of both the coagulation system and the complement system [33].
At the same time we find that an increased number of grafts, probably reflecting a better revascularization decrease the risk of a new ischemic event. A reduced number of coronary grafts could be patients with severe diffuse coronary artery disease thereby providing incomplete revascularization. The current study does not provide us with this information.
Limitations of the Study
The present study has some limitations. The study is observational without randomizations and prognostic factors may influence the treatment and decision-making and thereby bias estimates of treatment effects. Additionally, there is a risk of confounding by indication. Furthermore the method of propensity matching is highly dependent on the choice of variables following potential bias. In addition, a group of patients are excluded due to lack of match. However, since the included fraction of patients is relatively high this risk seems less important. The advantage in matching is that patients are compared equally. A randomized controlled trial is more ideal to control for unknown confounders but the propensity matching is an attempt to get as close as possible to compare groups similar in all ways except for continuing ASA/Clopidogrel. Furthermore, it is a disadvantage that the effect of Clopidogrel can only be investigated as a part of dual antiplatelet therapy and not alone.
An obvious limitation is that the patients, especially in the period up to 2013 with continued antiplatelet therapy are those who are exposed to acute surgery due to e.g. recent myocardial infarction, unstable angina pectoris or heart failure. However, as all the above mentioned is part of EuroSCORE risk evaluation and all are part of the matching model, the risk is attenuated. Further we encompassed into the model perioperative indicators of problematic foundations by including perioperative administration of vasoconstrictors and inotropes as found in previous studies [34,35].
Theoretically, not all patients with postoperative ischemic may be accounted for. However, with the tax-funded health system with equal and free access to the hospital system we consider this possibility as extremely low.
We do not know if there has been any preoperative events in the time from indication of surgery is made where medication maybe is started until surgery as this information cannot be extrapolated from our data. Further we do not know if patients have been cancelled and rescheduled. Another weakness of our data is that dead within 1-3 days postoperatively is not registered as a new ischemic event even though it might be the cause.
Conclusion
We found no increased risk of thromboembolic events for patients continuing their ASA in a few days due to cardiac surgery. Patients continuing ASA therapy carry a minor risk of increased postoperative bleeding. The risk is further increased if Clopidogrel is continued until the day before surgery. The optimal timing of AP therapy still needs solid evidence.
References
- Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005; 366: 1607-1621.
- Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK et al. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001; 358: 527-533.
- Jacob M, Smedira N, Blackstone E, Williams S, Cho L. Effect of timing of chronic preoperative aspirin discontinuation on morbidity and mortality in coronary artery bypass surgery. Circulation. 2011; 123: 577-583.
- Ebrahimi R, Dyke C, Mehran R, Manoukian SV, Feit F, Cox DA et al. Outcomes following pre-operative clopidogrel administration in patients with acute coronary syndromes undergoing coronary artery bypass surgery: the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. J Am Coll Cardiol. 2009; 53: 1965-1972.
- Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ et al. Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome. Circulation. 2004; 110: 1202-1208.
- Herman CR, Buth KJ, Kent BA, Hirsch GM. Clopidogrel increases blood transfusion and hemorrhagic complications in patients undergoing cardiac surgery. Ann Thorac Surg. 2010; 89: 397-402.
- Ferraris VA, Ferraris SP, Saha SP, Hessel EA 2nd, Haan CK Society of Thoracic Surgeons Blood Conservation Guideline Task Force, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann ThoracSurg. 2007; 83: 27-86.
- Windecker S Kolh P, Alfonso F, Collet JP, Cremer J, Falk V et al. 2014 ESC/ EACTS Guidelines on myocardial revascularization. Eur Heart J. 2014; 35: 2541-2619.
- Roffi M Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016; 37: 267-315.
- Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012; 33: 2569- 2619.
- Hastings S, Myles P, McIlroy D. Aspirin and coronary artery surgery: a systematic review and meta-analysis. Br J Anaesth. 2015; 115: 376-385.
- Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T Et al. ATACAS Investigators of the ANZCA Clinical Trials Network. Stopping vs. Continuing Aspirin before Coronary Artery Surgery. N Engl J Med. 2016; 374: 728-737.
- Schmidt M, Maeng M, Jakobsen CJ, Madsen M, Thuesen L, Nielsen PH et al. Existing data sources for clinical epidemiology: The Western Denmark Heart Registry. Clin Epidemiol. 2010; 2: 137-144.
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011; 39: 22-25.
- National Danish Transfusion-guidelines.
- Cao L, Young N, Liu H, Silvestry S, Sun W, Zhao N, et al. Preoperative aspirin use and outcomes in cardiac surgery patients. Ann Surg. 2012; 255: 399-404.
- Bybee KA, Powell BD, Valeti U, Rosales AG, Kopecky SL, Mullany C, et al. Preoperative aspirin therapy is associated with improved postoperative outcomes in patients undergoing coronary artery bypass grafting. Circulation. 2005; 112: I286-1292.
- Gielen CL, Bruggemans EF, Stijnen T, Eikenboom J, Tavilla G, Brand A, et al. Stopping antiplatelet medication before coronary artery bypass graft surgery: is there an optimal timing to minimize bleeding? Eur J CardiothoracSurg. 2015; 48: 64-70.
- Kulik A, Chan V, Ruel M. Antiplatelet therapy and coronary artery bypass graft surgery: perioperative safety and efficacy. Expert Opin Drug Saf. 2009; 8: 169-182.
- Benedetto U, Altman DG, Gerry S, Gray A, Lees B, Flather M, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial†. Eur J Cardiothorac Surg. 2017 Apr 6.
- Purkayastha S, Athanasiou T, Malinovski V, Tekkis P, Foale R, Casula R, et al. Does clopidogrel affect outcome after coronary artery bypass grafting? A meta-analysis. Heart. 2006; 92: 531-532.
- Ascione R, Ghosh A, Rogers CA, Cohen A, Monk C, Angelini GD. In-hospital patients exposed to clopidogrel before coronary artery bypass graft surgery: a word of caution. Ann ThoracSurg. 2005; 79: 1210-1216.
- Nijjer SS, Watson G, Athanasiou T, Malik IS. Safety of clopidogrel being continued until the time of coronary artery bypass grafting in patients with acute coronary syndrome: a meta-analysis of 34 studies. Eur Heart J. 2011; 32: 2970-2988.
- Bhaskar B, Dulhunty J, Mullany DV, Fraser JF. Impact of blood product transfusion on short and long-term survival after cardiac surgery: more evidence. Ann ThoracSurg. 2012; 94: 460-467.
- Jakobsen CJ, Ryhammer PK, Tang M, Andreasen JJ, Mortensen PE. Transfusion of blood during cardiac surgery is associated with higher longterm mortality in low-risk patients. Eur J CardiothoracSurg. 2012; 42: 114– 120.
- Firanescu CE, Martens EJ, Schonberger JP, Soliman Hamad MA, van Straten AH. Postoperative blood loss in patients undergoing coronary artery bypass surgery after preoperative treatment with clopidogrel. A prospective randomized controlled study. Eur J CardiothoracSurg. 2009; 36: 856–862.
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am CollCardiol. 2007; 49: 1505-1516.
- Ferraris VA, Saha SP, Oestreich JH, Song HK, Rosengart T, Reece TB, et al. 2012 update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann ThoracSurg. 2012; 94: 1761-1781.
- Plicner D, Mazur P, Hymczak H, Stoliński J, Litwinowicz R, Drwiła R, et al. Preoperative platelet aggregation predicts perioperative blood loss and rethoracotomy for bleeding in patients receiving dual antiplatelet treatment prior to coronary surgery. Thromb Res. 2015; 136: 519-525.
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/ STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016; 134: e123-155.
- Nocerino AG, Achenbach S, Taylor AJ. Meta-analysis of effect of single versus dual antiplatelet therapy on early patency of bypass conduits after coronary artery bypass grafting. Am J Cardiol. 2013; 112: 1576-1579.
- Demertzis S, Cassina T, Casso G. Antiplatelet therapy before cardiac surgery. Cardiovascular Medicine. 2016; 19: 110-116.
- Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg. 2011; 113: 1319-1333.
- Nielsen DV, Madsen M, Johnsen SP, Hansen M, Hindsholm K, Jakobsen CJ. Health Outcomes with and without use of Inotropic Therapy in Cardiac Surgery; Results of a Propensity-Score-Matched Analysis. Anesthesiology. 2014; 120(5): 1098-1108.
- Ryhammer PK, Tang M, Hoffmann-Petersen J, Leonaviciute D, Greisen J, Gissel MS, et al. Colloids in cardiac surgery – friend or foe. J CardiothoracVascAnesth. 2017 Feb 4. pii: S1053-0770(17)30051-4.
