Abstract
Introduction: Infective Endocarditis (IE) is a serious disease with a high mortality rate if not diagnosed early. Most patients are subjected to antimicrobial therapy alone with a good cure rate, but there is a group of more severely affected patients requiring an associated surgical approach. Pre-operative renal dysfunction is a marker of mortality, as well as worsening of renal function in surgical patients.
Objective: To evaluate preoperative and postoperative renal function, using serum creatinine as a marker of mortality, in surgical patients with IE.
Methods: We analyzed 59 consecutive patients undergoing cardiac surgery for the treatment of EI associated with antibiotic therapy in the period from Jan/2005 to Dec/2008. Serum creatinine was measured six times: on admission, the day before surgery, and 24h, 48h, 72h and 1 week postsurgery. Creatinine clearance was calculated from serum creatinine using the Cockroft-Gault formula. The variables were analyzed using ANOVA for repeated measures, Pearson correlation, and ROC curve; the alpha value used was 0.05.
Results: Repeated measurements showed that creatinine was higher in patients who died compared with those who survived (p=0.00608), which was not observed with creatinine clearance (p=0.24).
Conclusion: Renal dysfunction worsens the prognosis for patients undergoing cardiac surgery. Impaired renal function in the immediate postoperative period was an important predictor of death in these patients. Serum creatinine was a preoperative prognostic marker of death within 30 days after cardiac surgery for IE. However, creatinine clearance showed no prognostic value in this cohort.
Keywords: Endocarditis; Acute renal insufficiency; Cardiac surgical procedures; Valvular disease; Mortality
Introduction
Infectious endocarditis is a complex disease with several clinical presentations, and its prognosis worsens with delays in diagnosis and treatment. Is estimated the in hospital mortality rate as high as 30% [1-4].
It occurs mainly in the cardiac valves and presents high morbidity and mortality, and can be fatal if not diagnosed and treated adequately. Due the diversity of the clinical presentations, the disease has a large prognosis spectrum, ranging from resolution with no complications to development of congestive heart failure and sepsis, sometimes with need for inotropic support and intra-aortic balloon [1,2].
There are some patients in which isolated antibiotic therapy is not effective, and surgical approach is needed. All patients with refractory heart failure due to endocarditis should be considered for surgery, since the ventricular dysfunction in these patients is related to a poor prognosis when antimicrobial therapy is used alone [2-5].
In patients undergoing surgery, pre-operative renal function is a marker for mortality, and renal dysfunction in the immediate postoperative period also influences the prognosis.
The aim of this study was to evaluate the prognosis of patients undergoing cardiac surgery for the treatment of infective endocarditis and the role of renal function, represented by serum creatinine and by estimated creatinine clearance, as marker of in-hospital death.
Methods
This was a case study, time series, which analyzed the data in the medical records of all consecutive in-hospital patients of both genders with infective endocarditis that underwent cardiac surgery as an adjunctive treatment to antibiotics in the National Institute of Cardiology, Rio de Janeiro, Brazil, from January 2005 to December 2008. All patients were older than 18 years old.
The inclusion criteria were: patients with clinical, laboratory and echocardiographic infective endocarditis diagnosis by the Duke criteria [1]. Exclusion criteria were patients on renal dialysis prior to the episode of Infective Endocarditis.
The following variables related to exposure and outcomes were analyzed: a) demographics (age at diagnosis, gender and ethnicity, weight, height, Body Mass Index (BMI), and Body Surface Area (BSA)); b) history of previous heart disease, and congenital or acquired risk factors for cardiovascular disease; c) complete blood count; d) serum creatinine, measured six times: on admission, the day before surgery, and 24h, 48h, 72h and 1 week post-surgery; e) creatinine clearance calculated by the Cockroft-Gault formula; f) heart failure classified according to the New York Heart Association Criteria (NYHA); g) medicines used before surgery (antibiotics, digitalis, furosemide, thiazide, spironolactone, Angiotensin-Converting Enzyme (ACE) inhibitors, and aspirin); h) echocardiographic parameters (Left Atrium Dimension (LAD), Left Atrium Dimension/ Body Surface Area Relation (LAD/BSA), Left Ventricle Ejection Fraction (LVEF), Left Ventricular End Systolic Volume (LVESV), Left Ventricular End Diastolic Volume (LVEDV), Systolic Pulmonary Artery Pressure (SPAP), presence and quantification of valvular insufficiency or stenosis, vegetation dimension, type, and location); i) surgical parameters (time of Cardio Pulmonary Bypass (CPB) and aortic clamping, use of Intra-Aortic Balloon (IAB) and valve repair by valvuloplasty, or use of biological or mechanical prosthesis); j) postoperative parameters (mechanical ventilation time, need for reintubation, use of packed red cells, plasma, or cryoprecipitate); and k) outcome: death within 30 days after cardiac surgery.
Statistical analysis was carried out with the Statistica 8.0 software from Statsoft Inc. Descriptive continuous data were expressed as mean and Standard Deviation (SD) or median and quartiles as appropriate, and by percentages for categorical data. To discriminate differences between outcome groups we used the Student’s t-test for continuous variables and the chi-square or Fisher exact test as appropriate for categorical variables. The analysis of continuous variables measured along time was performed by Analysis of Variance (ANOVA) for repeated measures. We used an alpha value of 0.05. The project was approved by the Institutional Research Ethics Committee, accredited by the National Committee for Ethics in Research, number 0185/181207.
Results
During the study period 59 patients met the criteria for inclusion and exclusion. The demographic and laboratory characteristics of the population studied are summarized in (Table 1). Thirty-four patients (57.6%) were male. When analyzing age, the highest incidence was in the 3rd decade of life with a stable incidence from 4th to 6th decade followed by a significant decline. In relation to self-reported ethnicity, 57% of patients were identified as Caucasian, and 43% afro-Brazilian.
Mean
SD
Age (Years)
40.7
16.3
Weight (Kg)
62.7
12.9
Height (m)
1.67
0.09
BMI (Kg/m2)
22.4
3.7
BSA (m2)
1.70
0.19
Erythrocytes (106/ml)
3.76
0.89
Hematocrit (%)
31.2
7.0
Hemoglobin (g/dl)
10.05
2.89
Leukocytes (ml)
11,060
5,059
Creatinine (mg/dl)
1.00
0.36
Creatinine clearance (ml/min/1,73m2)
89.7
40.2
LAD (cm)
4.55
0.9
LA/BSA (cm/m2)
2.72
0.6
LVEF (%)
66.1
13.6
LVESV (ml)
68.7
81.5
LVEDV (ml)
162.8
67.2
SPAP (mmHg)
50.1
18.3
SD: Standard Deviation; BMI – Body Mass Index; BSA - Body Surface Area; LAD - Left Atrium Dimension; LAD/BSA - Left Atrium Dimension/Body Surface Area Relation, LVEF - Left Ventricular Ejection Fraction, LVESV - Left Ventricular End Systolic Volume; LVEDV - Left Ventricular End Diastolic Volume; and SPAP - Systolic Pulmonary Artery Pressure.
Table 1: Demographic and laboratory characteristics of the studied population.
The median time to endocarditis diagnosis in our institution was 0.5±16 days (-48 to 45 days) and the mean time of admission to surgery was 17±17 days (1-74 days). The median time to endocarditis diagnosis showed negative values because of the tertiary characteristics of our institution. Many patients arrived with a previous diagnosis and had been referred for surgical treatment.
In the clinical analysis, 78% (46) patients showed no identifiable bacteremia source. Eighty-eight percent (52) of patients had fever and malaise during the disease course, and 22% (13) had splenomegaly. Janeway stains were observed in 3.4% (2) patients and Osler nodules in only 1.7% (1) patient. Prior heart disease was identified in 71.1% of the patients - rheumatic heart disease in 50.8% (30), and other heart diseases in 20.3% (12).
Regarding the presence of risk factors for coronary disease, 5.1% (3) were diabetic, 20.4% (12) were former smokers, and 6.8% (4) were current smokers.
NYHA functional class greater than I, was presented in 88% (52) of patients; of these, 40% were in functional class III or IV. The median functional class was II, with interquartiles II and III. In the treatment of heart failure, 64.4% (38) required in-hospital diuretics, mainly furosemide, but only in 13.6% (8) of them, used associated spironolactone. The use of intravenous inotropic agents was necessary in only 6.8% (4) of the patients, and dobutamine was used in all of these cases.
Among the additional laboratory tests, the patients had erythrocytes counts of 3.76±0.89 (106/ml), hematocrit of 31.2±7.0% and hemoglobin of 10.05±2.89 g/dl. The leukocyte count was 11,060±5,059/ml. Serum creatinine on admission was 1.00±0.36 mg/ dl (0.5 to 1.94) and creatinine clearance estimated by the Cockroft- Gault formula was 89.7±40.2 ml/min/1,73m2 (Table 1).
A positive blood culture was observed in 66.1% (39) of our patients, with Streptococcus group accounting for 33.9% (20) of cases, representing the most frequently isolated germ. In this group, Streptococcus viridans was isolated in 85% (17/20) and Streptococcus bovis in 15% (3/20). The second most frequent group of bacteria was Staphylococcus, which accounted for 22.0% (13) of the isolated infectious endocarditis agents. In this group, Staphylococcus aureus was isolated in 46.1% (6/13) of the patients, Staphylococcus epidermidis in 38.5% (5/13) and Staphylococcus hominis in 15.4% (2/13).
Table 1 also summarizes the preoperative echocardiographic assessments: LAD (4.55±0.9 cm), LAD/BSA relation (2.72±0.6 cm/m2), LVEF (66.1±13.6%), LVESV (68.7±81.5 ml) and LVEDV (162.8+67.2 ml). The SPAP in the 33 patients presenting with tricuspid regurgitation, that allowed for measurement, was 50.1±18.3 mmHg.
In the topographic analysis of the primary lesion, we observed mitral valve lesion in 50.0% of cases, aortic valve lesion in 29.3%, and both mitral and aortic lesion in 13.8% of the patients. Among the overall study population, 28.8% (17) were patients with valve prosthesis. Seventeen percent of patients had more than one valve involved and only one patient (1.7%) showed no detectable vegetation on echocardiogram. Regarding vegetation, its largest diameter was 1.70±0.72 cm. The most frequent types were pedunculated in 33.9% (20) and sessile in 22.0% (13) of patients.
Postoperative complications were observed in 62.7% (37) of patients and reoperation was required in 27.1% (16), the majority (75% - 12/16) for homeostatic review. The length of ICU stay after cardiac surgery was 11±16 days (0-77), and total hospitalization length was 51±24 days (7-130).
The in-hospital mortality was 16.9% (10), sepsis being the leading cause of death (8.45%), followed by cardiogenic shock (3.4%) and multiple organ failure (3.4%). Only one patient died during surgery.
Univariate analysis of admission data showed a significant difference between the deceased and the survivor group for BMI, erythrocytes, hematocrit, hemoglobin, and mechanical ventilation length (Table 2). Serum creatinine at admission showed no significant difference between the two groups (Table 3).
Survival (49)
Deceased (10)
p
Mean
SD
Mean
SD
Age (Years)
39.28
15.86
47.42
17.51
0.1518
Weight (Kg)
61.75
12.15
67.21
16.02
0.2261
Height (m)
1.67
0.09
1.65
0.08
0.3844
BMI (Kg/m2)
21.94
3.48
24.56
4.35
0.0428
BSA (m2)
1.69
0.18
1.73
0.23
0.554
EuroSCORE points
5.38
3.15
7.3
4.16
0.1033
EuroSCORE logistic
0.088
0.093
0.161
0.197
0.0707
Total Cholesterol (mg/dl)
150.03
37.4
166.5
48.91
0.3494
Erythrocytes (106/ml)
3.86
0.87
3.24
0.88
0.0439
Hemoglobin (g/dl)
10.42
2.73
8.23
3.12
0.0281
Hematocrit (%)
31.97
6.85
27.19
6.7
0.0481
Leukocytes (ml)
10,560
4,212
13,461
7,906
0.099
LAD (cm)
4.51
0.93
4.76
0.74
0.4326
LA/BSA (cm/m2)
2.7
0.63
2.78
0.5
0.7131
LVEF (%)
66.98
13.15
62.1
15.77
0.306
LVESV (ml)
66.49
88.32
79.46
35.95
0.6688
LVEDV (ml)
157.47
68.09
188.44
60.01
0.2121
SPAP (mmHg)
49.24
18.5
56
18.56
0.4986
Vegetation diameter (cm)
1.69
0.76
1.729
0.44
0.9214
Perfusion time (min)
119.4
46.78
110.9
46.81
0.6022
Clamping Time (min)
104.14
46.75
98.1
42.04
0.7066
Ventilation time (hours)
70.75
221.33
302.5
488.39
0.0208
Table 2: Univariate analysis of demographic and laboratory characteristics between the deceased and the survivor group.
Survival (49)
Deceased (10)
p
Mean
SD
Mean
SD
Serum creatinine (mg/dl)
Admission
0.986
0.352
1.085
0.423
0.4379
Preoperative
1.001
0.319
1.278
0.607
0.0400
24h
0.888
0.419
1.358
0.637
0.0047
48h
0.992
0.523
1.402
0.737
0.0398
72h
0.999
0.478
1.531
0.797
0.0062
1 week
0.993
0.320
1.473
0.669
0.0009
Creatinine clearance (ml/min/1.73m2)
Admission
91.936
42.694
78.981
23.302
0.3576
Preoperative
89.074
40.615
69.335
24.830
0.1456
24h
113.962
66.491
69.207
31.547
0.0429
48h
103.578
56.226
80.576
57.847
0.2454
72h
100.117
59.619
64.658
31.054
0.0739
1 week
88.387
32.632
62.399
27.214
0.0221
Table 3: Univariate analysis of serum creatinine and creatinine clearance in relation to death.
Table 3 presents the univariate analysis for serum creatinine and creatinine clearance in relation to death and the six timeframes studied. The serum creatinine was higher in the deceased group all six timeframes studied. However, the performance of creatinine clearance was not identical. Only creatinine clearance that was performed at 24 hours of surgery and that at the end of the first week was significant.
When analyzing the renal function data sequence, it can be observed that the group of patients who expired had a progressive increase in serum creatinine compared to the group who survived (p=0.00608), where levels remained stable (Figure 1). In the analysis of creatinine clearance, this relationship was not observed (p=0.24) (Figure 2).
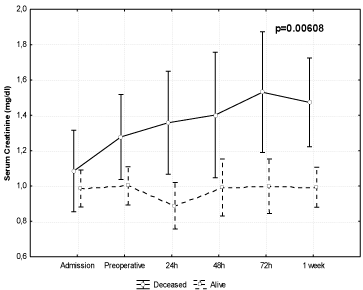
Figure 1: Analysis of variance for repeated measurements for serum
creatinine in relation to death.
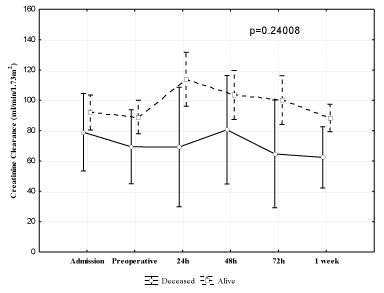
Figure 2: Analysis of variance for repeated measures for creatinine clearance
in relation to death.
The use of blood components in relation to death is shown in (Table 4). Only the amount of red blood cells and plasma units were higher postoperatively in the group who died.
Survival (49)
Deceased (10)
p
Mean
SD
Mean
SD
Preoperative
Erythrocytes
2.14
1.67
2.80
2.15
0.28514
Plasma
1.49
2.06
2.30
1.83
0.20971
Platelets
0.98
2.64
0.80
2.53
0.84436
Cryo-concentrated
1.96
3.35
2.70
4.19
0.54423
Postoperative
Erythrocytes
1.49
2.40
6.00
4.35
0.00002
Plasma
0.59
1.54
6.50
6.00
<0.00000
Platelets
0.63
2.08
2.20
3.42
0.05889
Cryo-concentrated
0.51
1.80
1.50
2.55
0.14717
Table 4: Use of blood components in the preoperative and postoperative care in relation to death.
Discussion
Infective endocarditis is a high-mortality disease if not properly treated. The surgical procedure is indicated in high-risk patients like uncontrolled infection, heart failure, presence of dysfunctional prosthetic valve or large vegetations with potential thromboembolism [1,2].
In this study, there were 57.6% male patients, a similar incidence with what is found the current literature [6-9]. We observed 42.6% of patients with a history of rheumatic fever. Because it is a population from a developing country in which rheumatic fever is still a reality, it is entirely plausible that rates of rheumatic fever sequelae are very different from those observed in developed countries.
Anemia is a frequent finding and a clinically significant prognostic marker in preoperative patients, and it is already established that anemic patients have a higher morbidity and postoperative mortality than non-anemic patients, in cardiac and non-cardiac surgery. This finding was observed by univariate analysis in our population [10- 13].
Leukocytosis has long been recognized as a marker of poor prognosis in patients hospitalized for infectious and noninfectious diseases, and is even more pronounced in specific etiologic agents [14-17]. In this study, the mean leukocyte count was 11,059. In Olaison et al.’s study, the mean leukocyte count was 11,200, and it was higher in patients with endocarditis due to Staphylococcus than in patients with endocarditis caused by Streptococcus viridans, and even higher in patients with complications related to endocarditis [14].
Serum creatinine was analyzed in some studies to evaluate its prognostic value for mortality risk in patients with infective endocarditis. A French study reported a 2mg/dl cutoff for death risk (RR=2.9 95% CI=1.80 to 4.53) [18].
Acute kidney injury is a complex syndrome that occurs in a range of clinical situations, and it may vary in presentation from a small alteration in serum creatinine to anuric renal insufficiency. It is a complication that occurs commonly in hospitalized patients and is an independent predictor of death [19-20]. Acute kidney injury can occur in up to 41.3% of patients after cardiac surgery and among this group, 9.6% of patients may eventually require dialysis, especially those patients with preoperative renal dysfunction [21].
As renal function changes, the mortality rises exponentially, from 1% in patients without renal disorders, to approximately 20% in patients with abnormal kidney function, and above 50% in patients requiring dialysis [22-25].
A major cause of acute kidney injury is postoperative cellular ischemia caused by hypotension [26]. Renal perfusion and glomerular filtration have self-regulated protective mechanisms that can be overtake when the mean arterial pressure fell lower than 80 mmHg [27]. The mean arterial pressure during cardiac surgery generally remains below that threshold, especially in cases of hemodynamic instability [28].
It is known that most patients undergoing cardiac surgery present comorbidities that lead to worsening of renal self-regulation, such as hypertension and diabetes mellitus. The use of medications that alter the ability to perform renal self-regulation, such as angiotensinconverting enzyme inhibitors and non-steroidal anti-inflammatory drugs, may explain this phenomenon [27].
Among the key predictors of acute kidney injury after surgery, the presence of previous renal dysfunction increases the risk of declining renal function after cardiac surgery, as well as diabetes mellitus, use of intra-aortic balloon in pre-surgery, preoperative anemia and reoperation procedure [29].
In this study, the change in postoperative creatinine was a predictor of mortality in relation to those who survived, regardless of prior renal function. Despite the observation that creatinine levels for each postoperative time were within normal limits in both groups, it is important to note the trajectory of values. In the survivor group, the serum creatinine remained, on average, unchanged. However, among the patients who died, these values tended to increase in postoperative follow-up. This is the main contribution of this work.
Very similar data were observed in the study conducted by Brown et al [30]. They observed that the increase in the postoperative creatinine greater than 50% added one seven times higher risk for death. However their series included only patients in postoperative coronary artery bypass surgery.
There were no statistically significant differences between creatinine clearance values. This observation may have several explanations. First, the values recorded in our study varied widely, especially in the deceased group, probably because it was a relatively small group. Creatinine clearance is calculated using serum creatinine, weight, age, and gender. Perhaps there are interactions among these four variables that were not detected in this work and could explain its lower power for deceased prediction.
In conclusion, kidney dysfunction worsens the prognosis of patients undergoing surgery. Associated with this, it was shown that worsening renal function in the immediate postoperative period was predictive of death in those patients. Serum creatinine was a preoperative prognostic marker of death within 30 days after surgery for infective endocarditis. However, creatinine clearance had not prognostic value in this cohort.
Limitations
Among the possible limitations of this study we wanted to emphasize the observational retrospective study design and the relatively small number of patients, although their number is in parallel with other observational studies. Analysis of renal dysfunction is also hampered by the inability to evaluate it before the episode of infective endocarditis, although this is an inherent problem in this setting. Another potential limitation was the inability to determine whether the renal dysfunction was due to glomerulonephritis, microembulus, or other complications during surgery. As the surgical treatment of infective endocarditis is only performed in patients with inappropriate clinical treatment response or with complications, it is clear that there is an increased risk of mortality in this subgroup.
References
- Baddour LM, Wilson WR, Bayer AS, Fowler VG, Bolger AF, Levison ME, et al. Infective Endocarditis: Diagnosis, Antimicrobial Therapy, and Management of Complications A Statement for Healthcare Professionals From the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association. Circulation. 2005; 111: 394-434.
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio- Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015; 21; 36: 3075-128.
- Leone S, Ravasio V, Durante-Mangoni E, Crapis M, Carosi G, Scotton PG, et al. Epidemiology, characteristics, and outcome of infective endocarditis in Italy: the Italian Study on Endocarditis. Infection. 2012; 40: 527-535.
- Olmos C, Vilacosta I, Fernandez C, Lopez J, Sarria C, Ferrera C, et al. Contemporary epidemiology and prognosis of septic shock in infective endocarditis. Eur Heart J. 2013; 34: 1999-2006.
- Yamaguchi H, Eishi K. Surgical Treatment of Active Infective Mitral ValveEndocarditis. Ann Thorac Cardiovasc Surg. 2007; 13: 150-55.
- Chu VH, Park LP, Athan E, Delahaye F, Freiberger T, Lamas C, et al. Association Between Surgical Indications, Operative Risk, and Clinical Outcome in Infective Endocarditis. Circulation. 2015; 131: 131-140.
- Aksoy O, Sexton DJ, Wang A, Pappas PA, Kourany W, Chu V, et al. Early Surgery in Patients with Infective Endocarditis: A Propensity Score Analysis. Clin Infec Dis. 2007: 44; 364-72.
- Hill EE, Herregods MC, VanderschuerenS, Claus P, Peetermans WE, Herijgers P. Outcome of Patients Requiring Valve Surgery During Active Infective Endocarditis. Ann Thorac Surg. 2008: 85; 1564-70.
- Gerrah R, Rudis E, Elami A, Milgalter E, Izhar U, Merin G. The Surgical Approach to Infective Endocarditis: 10 Year Experience. IMAJ. 2003: 5; 641- 5.
- Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996; 348: 1055-1060.
- Zindrou D, Taylor KM, Bagger JP. Preoperative haemoglobin concentration and mortality rate after coronary artery bypass surgery. Lancet. 2002; 359: 1747-1748.
- Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004; 5: 58S-69S.
- Ferraris VA, Ferraris SP. Risk factors for postoperative morbidity. J Thorac Cardiovasc Surg. 1996; 111: 731-738.
- Olaison L, Hogevik H, Kjell A. Fever, C-Reactive Protein, and Other Acute- Phase Reactants During Treatment of Infective Endocarditis. Arch Intern Med. 1997; 157: 885-89.
- De Campos T, Cerqueira C, Kuryura L, Parreira JG, Soldá S, Perlingeiro JA, et al. Morbimortality indicators in severe acute pancreatitis. JOP. 2008; 9: 690-7.
- Barreiro-López B, Tricas JM, Mauri E, Quintana S, Garau J. Risk factors and prognostic factors in nosocomial pneumonia outside the intensive care units setting. Enferm Infecc Microbiol Clin. 2005; 23: 519-24.
- Pesaro AE, Nicolau JC, Serrano CV Jr, Truffa R, Gaz MV, Karbstein R, Giraldez et al. Influence of leukocytes and glycemia on the prognosis of patients with acute myocardial infarction. Arq Bras Cardiol. 2009; 92: 84-93.
- Thuny F, Disalvo G, Belliard O, Avierinos JF, Pergola V, Rosenberg V, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005; 112: 69-75.
- Hashemzadeh K, Hashemzadeh S, Dehdilani M. Risk factors and outcomes of acute renal failure after open cardiac surgery. Asian Cardiovasc Thorac Ann. 2012; 20: 275-80.
- De Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, et al. Acute renal failure in the ICU: risk factors and outcome evaluation by SOFA score. Intensive Care Med. 2000; 26: 915-21.
- Yehia M, Collins JF, Beca J. Acute renal failure in patients with pre-existing renal dysfunction following coronary artery bypass grafting. Nephrology (Carlton). 2005; 10: 541-3.
- Kremneva LV, Abaturova OV, Shalaev SV. Rate of in-hospital cardiovascular complications in patients with postoperative renal dysfunction after surgical myocardial revascularization. Angiol Sosud Khir. 2016; 22: 124-129.
- Ostermann ME, Taube D, Morgan CJ, Evans TW. Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive Care Med. 2000; 26: 565-71.
- Bahar I, Akgul A, Ozatik MA, Vural KM, Demirbag AE, Boran M, et al. Acute renal failure following open heart surgery: risk factors and prognosis. Perfusion. 2005; 20: 317-22.
- Landoni G, Zangrillo A, Franco A, Aletti G, Roberti A, Calabro MG. Longterm outcome of patients who require renal replacement therapy after cardiac surgery. Eur J Anaesthesiol. 2006; 23: 17-22.
- Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002; 62: 1539- 1549.
- Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007; 357: 797-805.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006; 1: 19-32.
- Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute Kidney Injury After Cardiac Surgery: Focus on Modifiable Risk Factors. Circulation. 2009; 119: 495-502.
- Brown JR, Cochran RP, Dacey LJ, Ross CS, Kunzelman KS, Dunton RF, et al. Perioperative Increases in Serum Creatinine Are Predictive of Increased 90-Day Mortality After Coronary Artery Bypass Graft Surgery. Circulation. 2006; 114: 409-413.
