Abstract
Background: Left Ventricular Negative Remodeling (LVNR) following Primary Percutaneous Coronary Intervention (PPCI) is an important cause of LV systolic dysfunction due to Irreversible Myocardial Injury (IMI). Both necrosis and apoptosis contribute to IMI and LVNR. We assessed the role of specific apoptotic marker M30 in predicting LVNR in patients of anterior wall ST Elevation Myocardial Infarction (STEMI) undergoing PPCI within 12 hours of symptom onset.
Methods: This prospective study was done on 100 consecutive patients of anterior wall STEMI (87 men and 13 women, mean age 52.15±12.08 years) meeting our inclusion and exclusion criteria. Blood sample for M30 was drawn at 24 hours after symptom onset, when it reaches peak level. Transthoracic echo was done in each patient at 24 hours after PPCI and at 6 months. LVNR was defined as ≥20% increase in LV end diastolic volume at 6 months after PPCI.
Results: 44 patients (44%) developed LVNR at 6 months post PPCI. Diabetes mellitus (p=0.032), symptom onset to balloon time (p=0.059), CPK-MB (p=0.007) and M30 level (p=0.012) were independent predictors of LVNR. The cutoff value of M30 for predicting LVNR was 81.18u/ml with positive predictive value of 70.4% (AUC 85.3, p<0.001).
Conclusion: In patients of anterior wall STEMI undergoing PPCI, the apoptotic marker M30 is useful for early prediction of LVNR. This can assist in better risk stratification of patients after successful PPCI and identify the subgroup of patients who require more intensive medical follow up with antiremodeling drugs to attenuate the development of LVNR.
Keywords: Left ventricular negative remodeling, apoptotic biomarker; STelevation myocardial infarction; primary angioplasty percutaneous coronary intervention; CPK-MB; M30
Abbreviations
LVNR: Left Ventricular Negative Remodeling; IMI: Irreversible Myocardial Injury; PPCI: Primary Percutaneous Coronary Intervention; STEMI: ST-Elevation Myocardial Infarction; CPKMB: Creatine Phosphokinase-Myocardial Band; CK: Cytokeratin; LVEDV: Left Ventricular End Diastolic Volume; LAD: Left Anterior Descending Artery; TIMI: Thrombolysis in Myocardial Infarction; ACEI: Angiotensin Converting Enzyme Inhibitors; ARBs: Angiotensin Receptor Blockers; TTE: Trans-Thoracic Echocardiography; LVESV: Left Ventricular End Systolic Volume; LVEF: Left Ventricular Ejection Fraction; WMSI: Wall Motion Score Index; NT-ProBNP: N-Terminal-Pro-B-Type Natriuretic Peptide; ELISA: Enzyme Linked Immunosorbant Assay; SPSS: Statistical Package for the Social Science; SD: Standard Deviation; IQR: Inter- Quartile Range; ROC: Receiver Operating Characteristics; AUC: Area Under the Curve; BMI: Body Mass Index; GDMT: Guideline Directed Medical Therapy
Introduction
Left Ventricular Negative Remodeling (LVNR) i.e. progressive LV dilatation in spite of successful Primary Percutaneous Coronary Intervention (PPCI) is a major clinical problem in the modern era of ST-Elevation Myocardial Infarction (STEMI) management [1]. The most important predictor of LVNR following STEMI is the infarct size [2]. Both necrosis and apoptosis causing irreversible myocardial injury contribute to LVNR after STEMI [3]. The peak level of the necrotic biomarker Creatine Phosphokinase-Myocardial Band (CPKMB) following PPCI has been shown to be good predictor of infarct size and subsequent development of LVNR [4]. Turkoglu C, et al. [5] have shown that peak serum level of apoptotic factor M30 is also an independent predictor of LVNR at 6 months following PPCI. Both animal [6] and human studies [7] have shown that apoptosis occurs in 5-30% of cells in the infarct area during an acute coronary syndrome. Apoptosis and necrosis are morphologically distinct pathways leading to cardiomyocyte loss during ischemia and reperfusion [8].
During apoptosis, a number of intracellular proteins are cleaved by caspases. A neo-epitope in Cytokeratin (CK)-18, termed M30 antigen becomes available at an early caspase cleavage event during apoptosis and is not detected in vital or necrotic cells. A monoclonal antibody M30 specifically recognizes this M30 antigen. A fragment of CK18 (termed M65 antigen) is also released from cells during necrosis [9]. In accordance with other described studies [10], Senturk et al [11] and Turkoglu C et al [5] had assessed both M30 and M65 in patients of STEMI as marker of apoptosis and necrosis respectively. Turkoglu C et al [5] had shown M30 to be an independent predictor of LVNR in the European population, No study till date has been carried out in the Southeast Asian population to define the cut off value of M30 in this ethnic group. As the Body Mass Index (BMI) of our population is much lower than that of the studied European population, we carried out a pilot study to assess the cut off value of M30 in our population.
Methods
In this prospective study, 100 consecutive patients presenting with first episode of anterior wall STEMI within 12 hours of symptom onset to our emergency were studied. Flow chart of study design is shown in Figure 1. We have excluded the patients who were in (i) Killip class- IV, (ii) had presented with systolic blood pressure <90 mm Hg, (iii) presented with failed thrombolysis, (iv) had past history of myocardial infarction/ PCI/ coronary artery bypass surgery, (v) associated significant valvular heart disease, (vi) renal/liver failure, (vii) pregnancy, (viii) unprotected left main disease, (ix) flow in Left Anterior Descending artery (LAD) greater than TIMI-2, (x) expired within 24 hours of PPCI prior to echocardiographic evaluation for the study , (xi) poor transthoracic echo (TTE) window or (xii) had malignancy detected for which patients were under active treatment.
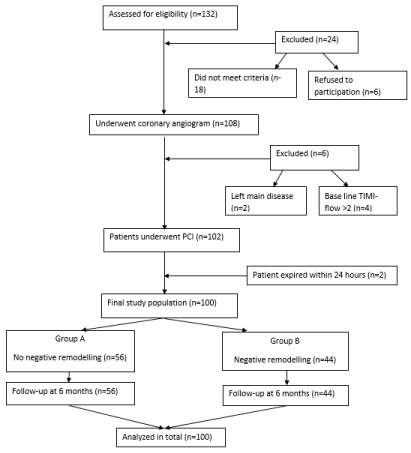
Figure 1: Flow chart of study design.
In our study, LVNR was defined as increase in left ventricle end diastolic volume (LVEDV) by ≥20% at 6 months after PPCI [12]. The patients were divided into two groups on the basis of 6 month post PPCI follow-up findings: non-LVNR group (Group A) and LVNR group (Group B). The study protocol was approved by the hospital ethical committee and each participant provided written informed consent. All the clinical details of the patients were recorded in a predesigned proforma.
PPCI
PPCI was performed in all patients within 12 hours of symptoms onset by experienced interventionists. Decision of thrombus aspiration, ballooning, stenting, and use of Glycoprotein (GpIIb/ IIIa) inhibitors was on operator’s discretion. Final Thrombolysis in Myocardial Infarction (TIMI)-3 flow in LAD with less than 20% residual stenosis was defined as successful PCI. The final TIMI-flow of less than 3 was defined as no-reflow in our study [13], which was assessed by two expert angiographers unaware of each other’s result or of the patient’s data. All patients were discharged on Guideline Directed Medical Therapy (GDMT) [14]. Angiotensin Converting Enzyme Inhibitors (ACEIs) or Angiotensin Receptor Blockers (ARBs) were continued if patient was already on therapy or initiated in all patients with systolic blood pressure greater than 100 mmHg. Beta blockers were similarly continued or initiated in all patients including patients with LVEF ≤40%, with no other contraindication for beta blocker therapy (reactive airway diseases, advanced AV block). Similarly aldosterone antagonists (eplerenone/spironolactone) were initiated in all patients with LVEF ≤40% in background of ACEIs/ ARBs and beta blocker therapy if they were diabetic or had symptoms of heart failure at presentation (Table 1).
Echocardiographic evaluation
All TTE initially and on follow-up were performed by an experienced blinded echocardiographer using PHILIPS ECHO machine (Model number UTAP20W) with 2.5-3.5 MHz transducer. All patients underwent TTE at 24 hours and at 6 months after PPCI. LVNR was defined as ≥20% increase in Left Ventricular End Diastolic Volume (LVEDV) at 6 months after PPCI based on repeated measurement in each patient and on the upper 95% confidence limit of the intraobserver variability [12]. In each patient the LVEDV, Left Ventricular End Systolic Volume (LVESV) and Left Ventricular Ejection Fraction (LVEF) were measured by the modified Simpson’s rule. The LV Wall Motion Score Index (WMSI) was assessed according to the definitions of the American Society of Echocardiography [15]. In order to assess intraobserver variability, echocardiographic parameters were measured offline in 30 patients a week after the first measurement. The intraobserver variability was calculated by difference between the two sets of measurements by the mean of the observations.
Assessment of biomarkers
Apart from routine biochemical investigations done on admission, biomarkers like M30, M65 and N-terminal-pro-B-type natriuretic peptide (NT-proBNP) were assessed at 24 hours from symptom onset. CPK-MB is the conventional biomarker of necrosis, which is routinely assessed in our institute. CPK-MB in the present study was assessed at 6 hourly intervals following PPCI till 24 hours as was done in other trials [4,16]. The peak value of CPK-MB amongst these four samples was taken as predictor of maximal myocardial necrosis. Apart from this, M30 (a marker of apoptosis), M65 (a marker of necrosis) and NT-proBNP (a marker of LV wall stress) were also assessed at 24 hours after symptom onset as peak level of M30 is achieved at this time following PPCI [10] with no significant change reported in peak level of M65 upto 48 hours [11].
M30 and M65 measurement: Blood samples were collected at 24 hours after symptom onset and were analyzed in duplicate in a blinded manner. The samples were centrifuged at 5000g for 10 minutes and serum aliquots were stored at -80C until analysis. Serum level of M30 and M65 were determined by commercially available immunoassays [M30-Apoptosense Enzyme Linked Immunosorbant Assay (ELISA) kit and M65 ELISA kit (Peviva AB, Bromma, Sweden)] according to manufacturer’s instructions. The M65 ELISA kit measures natural soluble CK18 (M65 antigen) whereas the M30 ELISA kit measures the level of CK18-Asp396 neoepitope (M30 antigen). Briefly the samples were placed in wells coated with a mouse monoclonal antibody as a catcher. After washing, a horse radish peroxidase conjugated antibody (M30 or M65) was used for detection. Reference concentration of M30 antigen or M65 antigen was used to prepare assay calibration. The absorbance was determined by an ELISA reader at 450 nm.
Follow-up
After discharge, patients were followed-up by hospital visits or telephonically at 1 and 3 months and a compulsory hospital visit at 6 months for final clinical evaluation and echocardiographic recording.
Statistical analysis
Statistical analysis was carried out using SPSS (Statistical Package for the Social Science) version 18 (SPSS, Inc, Chicago, Illinois, USA) software. Data are expressed as mean ± Standard Deviation (SD) or median with Inter-Quartile Range (IQR). Continuous variables were assessed by the Student t test and categorical variables by Mann- Whitney test. Correlation of the studied biomarker with various other biomarkers was assessed by Spearman’s correlation analysis. The univariate test was applied to assess the predictors that might be associated with LVNR. As number of subjects were small, only significant factors on univariate analysis (p<0.05) were selected for multivariate regression analysis. A logistic regression analysis was done to find out the independent predictors of LVNR. A stepwise backward likelihood method was applied for it. The optimal M30 cut off point for predicting LVNR was calculated using receiver operating characteristics (ROC) curve analysis. The Area Under the Curve (AUC) value was calculated as a measure of accuracy of the test. A two tailed p value of less than 0.05 was considered as statistically significant.
Results
Baseline clinical findings
There were 56 patients in Group A and 44 patients in Group B. No significant difference in age, sex, BMI and in risk factors like dyslipidemia, hypertension or smoking was seen between the two groups (Table 1). However, percentage of patients with diabetes mellitus (p=0.049) was significantly higher in Group B.
Variables
Group A (n=56)
Group B (n=44)
p –value
Age (years)
52.98±11.52
51.09±12.808
0.442
Systolic blood pressure (mmHg)
121.86±18.03
119.86±22.58
0.621
Diastolic blood pressure (mmHg)
81.18±9.34
77.86±9.11
0.322
Weight (Kg)
72.74 ±8.85
72.34±9.03
0.412
Height (cm)
171.55±3.67
171.43±3.64
0.462
BMI (Kg/m2)
24.76±2.85
24.64±2.90
0.418
Killip class
Killip-I
46 (82.14)
36 (81.81)
1
Killip-II
8 (14.28)
6 (13.63)
Killip-III
2 (3.57)
2 (4.54)
Hemoglobin (g/dl)
13.99±2.02
13.72±2.058
0.52
S. creatinine (mg/dl)
1.012±0.26
1.005±0.27
0.882
Blood urea (mg/dl)
29.72±12.40
25.61±7.78
0.069
Random blood sugar (mg/dl)
165.04±63.08
165.14±77.64
0.992
High density lipoprotein (mg/dl)
38.30±5.17
38.55±4.97
0.812
Total cholesterol (mg/dl)
172.98±30.17
173.39±30.63
0.942
Triglycerides (mg/dl)
204.11±42.99
190.73±45.43
0.135
Diabetes
4 (7.14)
9 (20.45)
0.049
Hypertension
10 (17.85)
13 (29.54)
0.162
Smoking
22 (46.4)
16 (31.6)
0.658
Medications at time of discharge
Aspirin
56 (100)
44 (100)
1
P2Y12inhibitors
56 (100)
44 (100)
1
Statins
56 (100)
44 (100)
1
ACEIs or ARBs
49 (87.5)
40 (90.9)
0.751
Beta blockers
50 (89.28)
41 (93.18)
0.727
Aldosterone antagonists
13 (23.21)
15 (34.09)
0.266
% of patients on GDMT or at least on 50% of target dose of GDMT
Aspirin
56 (100)
44 (100)
1
P2Y12inhibitors
55 (98.21)
43 (97.72)
1
Statins
54 (96.42)
44 (100)
0.502
ACEIs or ARBs
35 (62.5)
26 (59.09)
0.836
Beta blockers
40 (71.42)
30 (68.18)
0.826
Aldosterone antagonists
13 (23.21)
15 (34.09)
0.266
BMI=Body Mass Index, GDMT=Guideline Directed Medical Therapy. Values are mean±standard deviation or n (%).
Table 1: Baseline characteristics.
Angiographic and Interventional findings
The number of patients with symptom to balloon time of more than 6 hours was significantly higher in Group B compared to Group A (Table 2). There was no significant difference between the two groups with regard to stent diameter/number/length, use of thrombus aspiration, use of Glycoprotein IIb/IIIa inhibitors, incidence of concomitant multivessel disease along with baseline and residual Syntax score. Patients in group B had significantly higher percentage of patients with no-reflow than in Group A (Table 2).
Angiographic findings
Group A (n=56)
Group B (n=44)
p –value
Symptom onset to balloon time (>6hours) [n (%)]
19 (33.9)
29 (63.9)
0.001
Multivessel involvement [n (%)]
19 (33.92)
13 (29.54)
0.53
Total stent length (mm)
22.1±3.6
22.4±4.5
0.67
Stent diameter (mm)
3.3±0.29
3.4±1.1
0.56
Thrombus aspiration (%)
11 (19.64)
10 (22.72)
0.81
Glycoprotein IIb/IIIa inhibitors [n (%)]
8 (14.28)
8 (18.18)
0.78
Initial Syntax
16.9±5.1
17.8±4.4
0.55
Final Syntax
12.45±5.9
13.1±4.6
0.66
No-reflow [n (%)]
2(3.6)
8(18.2)
0.016
Table 2: Angiographic findings of the two groups.
Biomarkers of necrosis, apoptosis and LV wall stress
There was significant difference in the level of necrotic biomarker CPK-MB and M65, apoptotic biomarker M30 and NT-proBNP between Group A and B (Table 3).
Biomarker
Group A (n=56)
Group B (n=44)
p-value
CPK-MB [ng/ml]
162.0 [144.0-187.2]
240.0 [180.0-342.9]
<0.001
NT-proBNP [pg/ml]
258.5 [136.5-365.9]
490.0 [327.3-1014.0]
<0.001
M30 [U/l]
71.84 [58.45-100.63]
165.17 [93.68-285.7]
<0.001
M65 [U/l]
91.12 [78.66-404.0]
116.4 [96.7-404.0]
<0.001
Values are in median with interquartile range
Table 3: Biomarkers of necrosis,apoptosis and LV stress.
A very good correlation was found between the apoptotic marker M30 with necrotic biomarker M65, CPK-MB and NT-proBNP (Table 4). There was also a good correlation between M30 level and change in LV volume (baseline to 6 months) as assessed by echocardiography (Table 4).
Variable
Spearman's correlation (r)
p-value
M65
0.781
<0.001
CPK-MB
0.62
<0.001
NT-proBNP
0.454
<0.001
Symptom onset to balloon time
0.223
0.026
*Change in LV ESV
0.713
<0.001
*Change in LVEDV
0.789
<0.001
*Change in LVEF
0.085
0.535
*Change from baseline to 6 months, LVESV:Left Ventricular End Systolic Volume; LVEDV:Left Ventricular End Diastolic Volume, LVEF:Left Ventricular Ejection Fraction.
Table 4: Correlation of M30 with other variables.
Echocardiographic findings at baseline and on follow-up
A comparison of the echocardiographic findings indexed to body surface area is shown in Table 5. At baseline, there was no significant difference in LVEDV, LVESV and LVEF between the two groups. However, there was progressive increase in both LVEDV and LVESV in group B compared to the group A resulting in significant decrease in LVEF in group B patients (at the end of 6 months) compared to group A. The intra-observer variability in the evaluation of LVEDV and LVESV were 2.2±1.8 % and 2.9±2.1 % respectively.
Echocardiographic findings
Group A (n=56)
Group B (n=44)
p-value
LVEDV at baseline (ml/m2)
61.91±13.93
64.72±12.17
0.11
LVEDV at 6 month (ml/m2)
58.80±10.50
81.61±17.76
< 0.001
LVESV at baseline (ml/m2)
41.45±10.85
44.34±11.25
0.097
LVESV at 6 month (ml/m2)
37.42±11.36
58.80±14.69
<0.001
LVEF at Baseline (%)
43.25±5.6
40.45±9.7
0.06
LVEF at 6 months (%)
46±3.8
29±4.1
<0.001
Number of segments affected
3.2±1.3
3.7±2.1
0.07
LVEDV: Left Ventricular End-Diastolic Volume; LVESV=Left Ventricular End-Systolic Volume; LVEF: Left Ventricular Ejection Fraction; WMSI: Wall Motion Score Index.
Table 5: Echocardiographic findings.
Predictors of LVNR in patients with Anterior wall STEMI
In our study, the univariate test was first applied to predict the clinical and biochemical markers that may be associated with LVNR. Since numbers of subjects were small, only significant predictors (p≤0.05) at univariate analysis (Table 1,2 and 3) were selected for multivariate logistic regression analysis. The backward elimination model was applied to assess the independent predictors of LVNR. The bivariate correlation between M30 and M65 was 0.78 and it was collinear. Thus, M30 was included in the model (excluding M65) along with other biomarkers. The Hosmer-Leme show test for final model was applied for assessing the goodness of fit prediction model in multivariate analysis. Diabetes (p=0.032), symptom onset to balloon time (p=0.059), CPK-MB (p=0.007) and M30 (p=0.012) were found to be independent predictors of LVNR (Table 6).c
Variable
Coefficient (SE)
Odds ratio (95% CI)
P-value
Diabetes
2.11 (0.982)
8.259 (1.206-56.57)
0.032
Symptom onset to balloon time (> 6hours)
1.29 (0.682)
3.63 (0.954-13.82)
0.059
CPK-MB [ng/ml]
0.018 (0.007)
1.018 (1.005-1.032)
0.007
M30 [U/I]
0.016 (0.0016)
1.016 (1.003-1.028)
0.012
Table 6: Independent predictors of negative remodelling.
ROC curve analysis
The cutoff value of M30 for predicting LVNR on the basis of ROC curve (Figure 2) was 81.183 U/l with positive and negative predictive value of 70.4% and 87% respectively (Table 7). As CPK-MB is used in our hospital to assess the size of infarct and was also an independent predictor of LVNR, we constructed an ROC curve to predict LVNR by combining CPK-MB and M30. The optimal cutoff value of CPKMB was 122.5ng/ml with positive and negative predictive value of 84.8% and 76.1% (Table 7).
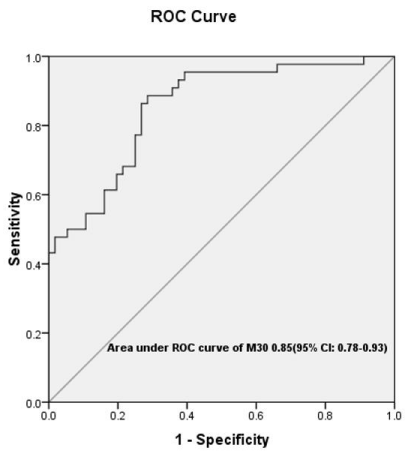
Figure 2: ROC curve for M30 biomarker.
Biomarker
AUC with 95% CI
p value
Sensitivity
Specificity
PPV
NPV
M30
85.3 [77.9-92.8
<0.001
88.63%
71.42%
70.40%
87%
CPK-MB
83.2 [75-91.5]
<0.001
63.60%
91.10%
84.80%
76.10%
M30+CPK-MB
87.3 [80.1-94.6]
<0.001
72.70%
91%
86.50%
81%
AUC:Area Under the Curve: CI:Confidence Interval; NPV:Negative Predictive Value; PPV:Positive Predictive Value
Table 7: Predictive value of various biomarkers.
Binary logistic regression analysis was used to assess the combined effect of CPK-MB and M30. The area under ROC of the combined biomarkers was 87.3 [95% Confidence Interval (CI); 80.1 to 94.6] with positive and negative predictive values of 86.5% and 81% respectively. The area under the ROC curve increased from 83.2 (for CPK-MB only) to 87.3 (for CPK-MB + M30) as shown in figure 3. The additional discriminate power of M30 was found to be statistically significant (p<0.001) using likelihood ratio test in binary logistic regression with CPK-MB only and with CPK-MB + M30.
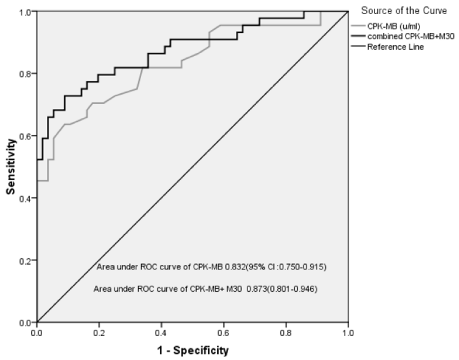
Figure 3: ROC curve to predict LV remodeling by combined CPK-MB and
M30.
Follow-up data
None of our patients who had survived for 24 hours following successful PPCI expired or were lost to follow-up. None of our patients during follow-up required re-hospitalization for recurrent coronary event, symptoms of heart failure or stroke. However, fourteen patients in LVNR group complained of worsening of dyspnoea at 3 months for which a loop diuretic had to be initiated. While eight patients improved symptomatically with one tablet of furosemide (40 mg), in six patients, dose had to be increased to two tablets daily after which they became asymptomatic till 6 months of follow-up. However, none of the patients required intravenous diuretics or hospital admission for worsening symptoms of heart failure. On further analysis, we found that six of these patients had no reflow following angioplasty, eight were diabetic and ten had symptom onset to balloon time of more than 6 hours. At end of 6 months, there was no significant difference in percentage of patients receiving GDMT between the two groups.
Discussion
To the best of our knowledge, this is the first study from Southeast Asia assessing the level of the apoptotic marker M30 in patients of STEMI undergoing PPCI to predict LVNR. We have used the same kit as used by Turkoglu et al. [5] in their study in the Turkish population to assess the cut-off value of M30 in a similar group of patients (first episode of anterior wall STEMI presenting within 12 hours of symptoms and undergoing PPCI) to predict LVNR. The cut-off value of M30 in the study by Turkoglu was significantly higher (144.9 U/l vs 81.183 U/l) in comparison to our study. This is due to the fact that the mean BMI of patients in their study was significantly higher 28.3 kg/m² (grade-1 obesity) [17] compared to mean of 24.64 kg/m² [overweight (BMI 23.0-24.9 kg/m²)] [18] in our study implying higher cardiac mass in the Turkish population than our studied population.
A study from Tiawan [19], has shown significantly higher M30 in healthy subjects with higher BMI (marker of obesity) than those with normal or lower BMI. Accordingly, the cutoff value of M30 in the patients of our study to predict LVNR was significantly lower than that of the study [5] in the Turkish population. Secondly in our study, the other biomarker which was independent predictor of LVNR was CPK-MB (a marker of volume of myocardial necrosis) but not NT-proBNP. The fact that a biomarker like CPK-MB was also independently associated with the changes in LVEDV demonstrate that our study had adequate power for detecting association of biomarkers depicting irreversible myocardial injury following myocardial infarction with LVNR.
Further due to a larger sample size, our study showed very good correlation of M30 with peak CPK-MB level unlike the study by Dincer Y et al. [20] who failed to show any correlation. In our study, NT-proBNP level assessed at 24 hours following PPCI failed to predict LVNR which is in agreement with the study by Heack DE et al. [21] who have shown that NT-proBNP assessed at 3 to 6 months is a better predictor of LVNR than that measured during the acute event in patients of anterior wall STEMI.
Apart from M30 (a marker of apoptosis), we had also assessed the level of M65 (a marker of necrosis) in our study. There was very good correlation between level of M30 and M65 in our study (r=0.781, p<0.001) showing that degree of necrosis parallels the degree of apoptosis following ischemic injury in patients of STEMI.
Clinical implication: Apart from well known factors like diabetes [22,23], symptom onset to balloon time [24] and peak CPK-MB level [25], M30 has also been found to be an independent predictor of LVNR in our study. In our study, both the group of patients had similar LVEF at time of discharge (Table 5) but Group B patients due to LVNR had significantly lower ejection fraction at the end of 6 months. The percentage of patients who were on recommended dose or at least 50% of dose of GDMT at six month were similar (Table 1). This shows the limitation of present medical therapy in preventing LVNR. There have been animal studies where anti-apoptotic agents have been shown to attenuate the extent of LVNR [26,27] but no definitive therapy targeting apoptosis has yet evolved.
Limitations
• Single centre study with small sample size which needs to be confirmed in larger studies.
• LVNR was assessed by echocardiography while cardiovascular magnetic resonance imaging is considered the gold standard.
Conclusion
LVNR can be predicted early after acute STEMI by assessing the apoptotic biomarker M30 along with conventional biomarker of necrosis like CPK-MB. This can assist in better risk stratification of patients after successful PPCI and identify the subgroup of patients who require more intensive medical follow up with anti-remodeling drugs to attenuate the development of LVNR.
Acknowledgement
We are thankful to Dr Rajeev Kumar Malhotra, scientist (statistician), Delhi cancer registry, Dr B.R.A. IRCH, AIIMS, Delhi for statistical analysis.
References
- Fertin M, Bauters A, Pinet F, Bauters C. Circulating levels of soluble Fas ligand and left ventricular remodeling after acute myocardial infarction (from the REVE-2 study). J Cardiol. 2012; 60: 93-97.
- Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993; 87: 755-763.
- Takemura G, Ohno M, Hayakawa Y, Misao J, Kanoh M, Ohno A, et al. Role of apoptosis in the disappearance of infiltrated and proliferated interstitial cells after myocardial infarction. Circ Res. 1998; 82: 1130-1138.
- Bagai A, Schulte PJ, Granger CB, Mahaffey KW, Christenson RH, Bell G, et al. Prognostic implications of creatine kinase-MB measurements in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. Am Heart J. 2014; 168: 503-11.e2.
- Türkoğlu C, Gür M, Şeker T, Selek Ş, Koçyiğit A. The predictive value of M30 and oxidative stress for left ventricular remodeling in patients with anterior STsegment elevation myocardial infarction treated with primary percutaneous coronary intervention. Coron Artery Dis. 2016; 27: 690-695.
- Bialik S, Geenen DL, Sasson IE, Cheng R, Horner JW, Evans SM, et al. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest. 1997; 100: 1363-01372.
- Baldi A, Abbate A, Bussani R, Patti G, Melfi R, Angelini A, et al. Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol. 2002; 34: 165-174.
- Baxter GF, Yellon DM. Current trends and controversies in ischemiareperfusion research-Meeting report of the Hatter Institute 3rd International Workshop on Cardioprotection. Basic Res Cardiol. 2003; 98: 133-136.
- Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004; 214: 1-9.
- Ueno T, Toi M, Linder S. Detection of epithelial cell death in the body by cytokeratin 18 measurement. Biomed Pharmacother. 2005; 59: S359-S362.
- Senturk T, Aydinlar A, Yilmaz Y, Oral AY, Ozdabakoglu O, Ulukaya E. Serial changes in circulating M30 antigen, a biomarker of apoptosis, in patients with acute coronary syndromes: relationship with the severity of coronary artery disease. Coron Artery Dis. 2009; 20: 494-498.
- Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002; 106: 2351-2357.
- Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009; 54: 281-292.
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with STsegment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018; 39: 119-177.
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28: 1-39.e14.
- Carvalho G, RassiS. The Prognostic Value of CK-MB in Acute Myocardial Infarction in Developing Countries. Angiol. 2016; 4: 183-189.
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000; 894: 1-253.
- The Asia Pacific Perspective-Redefining obesity and it’s treatment. Geneva. World Heath Organization. 2000; 17-18.
- Yang MC, Liu HK, Su YT, Tsai CC, Wu JR. Serum apoptotic marker M30 is positively correlated with early diastolic dysfunction in adolescent obesity. PLoS One. 2019; 14: e0217429.
- DincerY, Himmetoglu S, Bozcali E. Serum level of M30 Antigen in Acute Myocardial Infarction. Focus on Sciences. 2008; 1: 1-4.
- Haeck JD, Verouden NJ, Kuijt WJ, Koch KT, Van Straalen JP, Fischer J, et al. Comparison of usefulness of N-terminal pro-brain natriuretic peptide as an independent predictor of cardiac function among admission cardiac serum biomarkers in patients with anterior wall versus nonanterior wall ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2010; 105: 1065-1069.
- Funaro S, La Torre G, Madonna M, Galiuto L, Scarà A, Labbadia A, et al. Incidence, determinants and prognostic value of reverse left ventricular remodelling after primary percutaneous coronary intervention. Results of the Acute Myocardial Infarction Contrast Imaging (AMICI) multicenter study. Eur Heart J. 2009; 30: 566-575.
- Von Bibra H, St John Sutton M. Impact of diabetes on postinfarction heart failure and left ventricular remodeling. Curr Heart Fail Rep. 2011; 8: 242-251.
- Farag EM, Al-Daydamony MM. Symptom-to-balloon time and myocardial blush grade are predictors of left ventricular remodelling after successful primary percutaneous coronary intervention. Cardiovasc J Afr. 2017; 28: 186- 190.
- Hsu JT, Chung CM, Chu CM, Lin YS, Pan KL, Chang JJ, et al. Predictors of Left Ventricle Remodeling: Combined Plasma B-type Natriuretic Peptide Decreasing Ratio and Peak Creatine Kinase-MB. Int J Med Sci. 2017; 14: 75-85.
- Hayakawa K, Takemura G, Kanoh M, Li Y, Koda M, Kawase Y, et al. Inhibition of granulation tissue cell apoptosis during the subacute stage of myocardial infarction improves cardiac remodeling and dysfunction at the chronic stage. Circulation. 2003; 108: 104-109.
- Heidrich FM, Ritzkat A, Poitz DM, Ebner A, Cremers MM, Ruf TF, et al. A Novel Mechanism to Reduce Myocardial Remodeling: The Nitric Oxide Synthase Inhibitor L-NAME Shields the Remote Myocardium from Apoptosis and Fibrosis after Myocardial Infarction in-vivo. Int J Clin Cardiol Res. 2017; 1: 20-30.
