Abstract
Background: Previous studies have shown that high-sensitivity C-reactive protein (hs-CRP) was an independent risk factor for Contrast-Induced Acute Kidney Injury (CI-AKI). However, the relationship between new inflammatory indicators (eg. NLR, Neutrophil to Lymphocyte Ratio; MLR, Monocyte to Lymphocyte Ratio; PLR, Platelets to Lymphocyte Ratio) and CI-AKI remains unclear.
Methods: It was a multicenter retrospective observational study. Patients undergoing elective percutaneous coronary angiogram with creatinine record pre- and post-operative in 72hrs were recruited into this study from January 2015 to December 2019. All patients were divided into CI-AKI and non-CI-AKI groups. Multivariate logistic regression was used to explore the predictive value of inflammation indicators on CI-AKI. The receiver operating characteristic (ROC) curve was used, and the area under the ROC curve (AUC) were calculated.
Results: Totally 3545 patients were enrolled, and 15.0% (532/3545) patients suffered from CI-AKI. Multivariate logistic regression analysis indicated that hs-CRP (OR 1.025, 95% CI 1.014-1.036, P<0.001) was an independent risk factor for the incidence of CI-AKI. NLR (OR 1.121, 95% CI 1.078-1.165, P<0.001), MLR (OR 5.672, 95% CI 3.400-9.463, P<0.001), PLR (OR1.043, 95% CI 1.024-1.062, P=0.001) were also independent risk factors for the incidence of CI-AKI. All the results were confirmed in the subgroup analysis, and the results were consistent.
Conclusions: Elevated levels of inflammatory indicators, including hs-CRP, NLR, MLR, and PLR are independent risk factors for the incidence of CI-AKI in patients undergoing percutaneous coronary angiogram.
Keywords: Contrast-induced acute kidney injury; Inflammatory indicators; Neutrophil to lymphocyte ratio; Monocyte to lymphocyte ratio; Platelets to lymphocyte ratio; Percutaneous coronary angiogram
Introduction
Coronary Artery Disease (CAD) which has a mortality rate of approximately 13%, ranks as the third leading cause of death in China. Coronary Angiography (CAG) and Percutaneous Coronary Intervention (PCI), which are the gold standard for vascular assessment and effective therapy for CAD patients, are widely used. However, complications of these procedures have been gradually brought to attention. Contrast-Induced Acute Kidney Injury (CIAKI) remains one of the most common complications of these procedures, which is significantly associated with prolonged hospitalization, medical expense and increased short-term or longterm mortality [1,2]. It was found that the incidence of CI-AKI in patients with serum creatine (Scr) >176umol/L before CAG occurs in up to 20-30% [3], and is generally considered to be the third most common cause of in hospital AKI [4,5].
The pathogenesis of CI-AKI has not yet been fully clarified. It was reported that decreased renal blood perfusion and renal tubular epithelial damage caused by oxygen free radicals will lead to renal medullary ischemia hypoxia damage [6-9]. The occurrence of CI-AKI might be associated with decreased coronary blood flow, hemodynamic instability, renal micro-thrombosis, inflammatory impairs, drug toxicity and other factors [10,11]. However, there are no recognized particularly effective preventive measures of CI-AKI except volume expansion, according to the latest European Society of Urogenital Radiology (ESUR) guidelines. It is a necessity and feasibility to further explore the underlying mechanism of CI-AKI [5,12,13].
Complete blood count (CBC) is a simple, inexpensive, and routine examination that can provide us with a wealth of bloodrelated information, including the number and size of Red Blood Cells (RBCs), White Blood Cells (WBCs), Platelets (PLTs) and other cell subgroups. Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR), and Platelets to Lymphocyte Ratio (PLR) have been proposed as surrogate indicators of endothelial dysfunction and inflammatory response, and has prognostic values [14-16]. Studies showed that NLR was related to the incidence and mortality of STEMI, NSTEMI, and acute cerebral infarction [17,18]. Elevated levels of PLR reflect inflammation status, atherosclerosis and platelet activation[19]. Recently, some studies found that PLR level in patients with CI-AKI were significantly higher than that in patients without CI-AKI after PCI or CAG. This indicated that elevated PLR might be a potential predictor of CI-AKI [20-24]. PLR has also been proven to be an inexpensive and convenient method to predict the occurrence of CI-AKI after PCI or CAG in patients with Acute Coronary Syndrome (ACS) [20-24]. However, the relationship between PLR and CI-AKI in chronic coronary syndrome remains unclear. MLR also serves as a highly stable composite inflammatory index. However, few studies to explore the relationship between MLR and CI-AKI, and rare study to include NLR, PLR, and MLR simultaneously.
The current study evaluates the inflammation indicators (hsCRP, NLR, MLR and PLR) before elective percutaneous coronary angiogram, and to explore the early predictive value of inflammation indicators for CI-AKI.
Methods
Study Design and Setting
All consecutive eligible patients underwent elective percutaneous coronary angiogram were retrospectively recruited into this study from January 2015 to December 2019 at Sir Run Run Shaw Hospital and its medical consortium hospitals. Exclusion criteria were: (1) patients with Contrast Media (CM) use within 1 week before PCI (2) patients with nephrotoxic drugs use within 2 weeks; (3) allergic to CM; (4) patients with pre-existing end stage renal disease requiring hemodialysis, eGFR (estimated glomerular filtration rate) <45 mL/ min/1.73m2; (5) patients with Non ST segment Elevation Myocardial Infarction (NSTEMI), ST Segment Eelevation Myocardial Infarction (STEMI) or high-risk unstable angina pectoris (UA) within 1 month; (6) patients with cardiogenic shock, stroke or severe valvular heart disease. The study was approved by the Medical Ethical Review Committee of Sir Run Run Shaw Hospital (NO.20201217-36). Informed consent for experiments involving human samples was obtained from all participants.
Procedures and Definitions
The data were collected from Hospital Information System (HIS), included the demographic information including age, gender, Body Mass Index (BMI), comorbid diseases, and current used medications. Results of laboratory blood biochemical tests, type and volume of CM during the procedure were documented. CBC was measured in all patients at hospital admission. Automatic Blood Cell Counter (XE- 2100, Sysmex, Kobe, Japan) was used to measure Neutrophil (N), Lymphocyte (L), Monocyte (M), Platelet (PLT) counts and calculated NLR, PLR, MLR. Scr concentrations were measured in all patients at hospital admission, and the postoperative Scr concentrations recorded were the highest level measured at least 3 times within a 72- hour timeframe. An increase of either 25% or 0.5mg/dL (44.2μmoI/L) in basal Scr level within 72 hours following the implementation of CM was identified as CI-AKI [25]. Based on the diagnose of CI-AKI, patients were divided into CI-AKI and non-CI-AKI groups. The treatment strategies and perioperative medications were based on the current guidelines. Iodine CM (iohexol, iopamidol, iodixanol) was used during procedure. The formula invented by Cigarroa was used to calculate the dose of CM: 5ml×weight (Kg)/Cr(mg/dl), and maximum dose < 300ml. CM overuse was defined if dose exceeded above.
Data Analysis
All tests were performed using the SPSS statistical package, version 24.0 (Chicago, Illinois, USA). Continuous variables are presented as mean±SD if normally distributed, or as median (interquartile range) if not, and compared using t test or non-parametric Mann-Whitney U test. Categorical variables were expressed as numbers (percentage) and compared with chi-square test or Fisher exact test. Logistic regression analysis was used to explore the independent predictors of CI-AKI. The Area Under the Curve (AUC) of inflammatory factors was evaluated by Receiver Operating Characteristic (ROC) curve analysis. P-values < 0.05 were considered statistically significant unless stated otherwise.
Results
Baseline Characteristics of the Population
A total 3545 patients were enrolled in the current study, 15.01% (532/3545) were diagnosed as CI-AKI. The baseline clinical and procedural characteristics are shown in (Table 1). Compared with non-CI-AKI group, those with CI-AKI were significantly older (70 vs 67, p < 0.001), more female patients (43.67% vs 34.38%, p < 0.001), more diabetes (p < 0.001) and more CM overuse (6.3% vs 4.0%, p=0.022). In the group of CI-AKI, patients had higher BMI, and lower triglyceride, hemoglobin and Estimated Glomerular Filtration Rate (eGFR) (p for all <0.05). In addition, patients with CI-AKI had higher level of inflammatory indicators, including NLR, MLR, PLR, and hs-CRP (p for all <0.05).
Variable
Non-CI-AKI
n = 3013CI-AKI,
n = 532p
Age (years)
66.8±10.4
70.1±9.9
<0.001
Male, N(%)
1977(65.6)
305(57.3)
<0.001
BMI
24.4±5.3
25.2±5.4
0.005
Current smoking, N(%)
469(15.6)
69(13.0)
0.132
DM, N(%)
696(23.1)
150(28.2)
0.013
Hypertension, N(%)
1921(63.8)
354(66.5)
0.221
Previous MI, N(%)
45(7.9)
8(7.5)
0.551
Previous PCI, N(%)
159(27.4)
23(21.5)
0.234
Stable angina Pectoris, N(%)
925(30.7)
105(19.8)
0.046
EF(%)
60.88±12.21
58.6±10.97
0.111
eGFR(ml/min/1.73m^2)
84.53(67.33-94.43)
82.04(56.1-94.26)
<0.001
Laboratory index
WBC (×10^9/L)
6.2(5.1-7.3)
6.35(5-7.7)
0.045
lymphocyte (×10^9/L)
22.9(17.7-28.6)
19.525(14.4-25.9375)
<0.001
Mononuclear I cells (×10^9/L)
7.3(6.05-8.8)
7.5(6.1-9.4)
0.03
neutrophil (×10^9/L)
66.5(60.4-72.1)
69.2(62.5125-75.9)
<0.001
PLT (×10^9/L)
172(139-212)
166(135.125-210)
0.36
NLR
2.89(2.12-4.03)
3.62(2.38-5.25)
<0.001
MLR
0.32(0.24-0.44)
0.39(0.28-0.55)
<0.001
PLR
7.51(5.62-10.5)
8.41(6.04-12.83)
<0.001
hs-CRP (mg/L)
1.8(0.8-5)
3.7(1.3-11.025)
<0.001
LDL-C (mmol/L)
2.2±0.9
2.18±0.92
0.668
HDL-C (mmol/L)
0.99(0.84-1.18)
0.98(0.78-1.17)
0.021
Total cholesterol (mmol/L)
4.12±1.18
4±1.2
0.027
Triglyceride (mmol/L)
1.29(0.97-1.825)
1.18(0.86-1.59)
<0.001
Medicine
ACEI, N (%)
459(15.2)
99(18.6)
0.053
CCB, N (%)
882(29.3)
166(31.2)
0.381
Statin, N (%)
2568(85.2)
396(74.4)
<0.001
Contrast volume>5ml×weight (Kg)/Cr(mg/dl)
108(4.0)
31(6.3)
0.022
BMI-Body Mass Index; EF-Ejection Fraction; PLT-Platelets; NLR-Neutrophil To Lymphocyte Ratio; MLR-Monocyte To Lymphocyte Ratio; PLR-Platelets To Lymphocyte Ratio; hs-CRP-High-Sensitivity C-Reactive Protein; LDL-C-Low-Density Lipoprotein Cholesterol; HDL-C-High-Density Lipoprotein Cholesterol; ACEI-Angiotensin Converting Enzyme Inhibitor; CCB-Calcium Calcium Entry Blockers; eGFR-Esti Mated Glomerularfiltrationrate; DM-Diabetes Mellitus
Table 1: Basic clinical and procedural characteristics of the CI-AKI and Non–CIAKI groups.
Regression Analysis
Univariate logistic analysis showed in (Table 2) was performed, and totally 11 variables were significantly associated with CI-AKI, including elderly, female, diabetes, BMI, LVEF, eGFR, dose of CM and 4 inflammatory factors (NLR, MLR, PLR, hs-CRP).
Variable
Univariate analysis
OR
95%CI
p
Age (per 1years)
1.034
1.024-1.044
<0.001
Male
0.704
0.584-0.849
<0.001
BMI
1.028
1.009-1.048
0.004
Current smoking
0.808
0.616-1.060
0.125
DM
1.307
1.063-1.608
0.011
Hypertension
1.131
0.93-1.347
0.217
EF<35%
2.622
2.173-3.163
<0.001
eGFR<60
1.891
1.5301-2.337
<0.001
CRP (per 1mg/L)
1.035
1.027-1.044
<0.001
LDL-C (per 1mmol/L)
0.978
0.882-1.084
0.668
NLR
1.139
1.104-1.175
<0.001
MLR
6.828
4.561-10.221
<0.001
PLR
1.051
1.036-1.066
<0.001
Contrast
volume>5ml×weight (Kg)/Cr(mg/dl)1.629
1.08-2.459
0.02
BMI-Body Mass Index; EF-Ejection Fraction; PLT-Platelets; NLR-Neutrophil To Lymphocyte Ratio; MLR-Monocyte To Lymphocyte Ratio; PLR-Platelets To Lymphocyte Ratio; hs-CRP-High-Sensitivity C-Reactive Protein; LDL-C-Low-Density Lipoprotein Cholesterol; HDL-C-High-Density Lipoproteincholesterol; eGFR-Esti Mated Hlomerularfiltrationrate; DM-Diabetes Mellitus
Table 2: Univariate analysis for the risk factors of CI-AKI in patients who underwent PCI.
Multivariate logistic analysis showed in (Table 3) was performed to explore independent risk factors for CI-AKI. After adjusting for age, gender, diabetes, BMI, LVEF, eGFR, dose of CM and other potential confounders, high level of hs-CRP (OR1.025, 95% CI 1.014-1.036, p<0.001) is an independent risk factor for CI-AKI. Similarly, NLR (OR1.121, 95% CI 1.078-1.165, p<0.001), MLR (OR 5.672, 95% CI 3.4-9.463, p<0.001) and PLR (OR1.043, 95% CI 1.024-1.062, p=0.001) are independent risk factors for CI-AKI.
CRP
NLR
MLR
PLR
OR
95%CI
p
OR
95%CI
p
OR
95%CI
p
OR
95%CI
p
CRP/NLR/MLR/PLR
1.025
1.014
1.036
0
1.121
1.078
1.165
0
5.672
3.4
9.463
0
1.043
1.024
1.062
0
Age (Per 1 Years
1.026
1.014
1.039
0
1.024
1.012
1.037
0
1.02
1.007
1.032
0.002
1.028
1.015
1.04
0
Male
0.71
0.562
0.898
0.004
0.685
0.541
0.868
0.002
0.621
0.488
0.793
0
0.71
0.561
0.898
0.004
BMI
1.011
0.99
1.032
0.325
1.01
0.989
1.032
0.335
1.01
0.988
1.031
0.372
1.011
0.99
1.032
0.322
DM
1.32
1.029
1.694
0.029
1.301
1.013
1.67
0.039
1.347
1.049
1.73
0.02
1.3
1.013
1.668
0.039
Hypertension
954
0.751
1.213
0.702
0.957
0.752
1.218
0.722
0.981
0.771
1.249
0.877
0.931
0.733
1.184
0.56
EF>35%
2.525
2.013
3.167
0
2.66
2.123
3.333
0
2.608
2.08
30269
0
2.66
2.125
3.331
0
eGFR<60
1.164
0.876
1.547
0.294
1.111
0.835
1.479
0.469
1.092
0.82
1.455
0.546
1.14
0.858
1.514
0.367
Contrast volume >5* weight
1.151
0.693
1.912
0.586
1.081
0.65
1.799
0.764
1.023
0.614
1.705
0.93
1.096
0.659
1.823
0.725
PLT-Platelets; NLR-Neutrophil To Lymphocyte Ratio; MLR-Monocyte To Lymphocyte Ratio; PLR-Platelets To Lymphocyte Ratio-hs-CRP-high-sensitivity C-reactive protein; Contrast-volume>5ml×weight (Kg)/Cr(mg/dl)
Table 3: Multivariate analysis for the risk factors of CI-AKI in patients who underwent PCI.
Subgroup Analysis
Subgroup analysis in age, sex, hypertension, diabetes, renal insufficiency, and heart failure were performed. The results showed that CRP, NLR, MLR and PLR have predictive values for CI-AKI except for the value of PLR in the population of female and patient without hypertension (Figure 1).
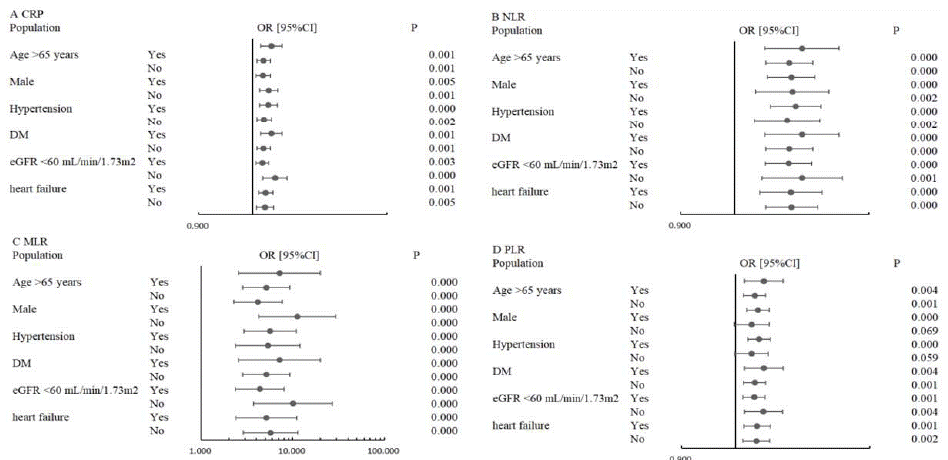
Figure 1: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
ROC Curve Analysis
The AUC of hs-CRP is 0.618 (95% CI: 0.592-0.645, P<0.001). The AUC of NLR, MLR and PLR respectively are 0.606 (95% CI: 0.578- 0.633, P<0.001), 0.604 (95% CI: 0.577-0.631, P<0.001), 0.570 (95% CI: 0.542-0.598, P<0.001).
Discussion
As the procedure of CAG and PCI widely applied in the diagnosis and treatment of CAD, CI-AKI has become one of the most severe complications. Although there is no effective treatment for CI-AKI [26-30], it is feasible to prevent CI-AKI. It is relatively rare research to explore the relationship between inflammatory indicators and the incidence of CI-AKI in elective percutaneous coronary angiogram. This retrospective study confirmed that inflammatory indicators including hs-CRP, NLR, MLR, and PLR were independent risk factors for CI-AKI in patients undergoing elective percutaneous coronary angiogram, which were confirmed in the sensitivity analyses.
The pathophysiological processes of CI-AKI are very complex and remain unclear. It was reported that renal ischemia and nephrotoxicity induce functional and structural changes of renal tubular epithelial cells and renal vascular endothelial cells, and then cause CI-AKI. Furthermore, inflammation is also considered as one of the basic mechanisms of CI-AKI [4]. It was reported that CM accelerate the production of oxygen free radicals, which leads to an inflammatory response. Then inflammatory factors (such as neutrophils, macrophages, natural killer cells, lymphocytes, etc.) infiltrate the damaged tissues and induce inflammatory mediators, including cytokines and chemokines. All of these might contribute to damage the renal vascular endothelium, which could aggravate renal function damage [31,32]. Therefore, inflammation may play an important role in the initial and subsequent stages of CI-AKI.
Hs-CRP is currently regarded as the main inflammatory factor in clinical, which is directly related to the inflammatory status. Elevated levels of hs-CRP can lead to increased expression of adhesion molecules, reduced nitric oxide production and decreased antioxidant defense capabilities, which cause endothelial dysfunction [33]. The blood vessels are prone to pro-thrombotic, pro-inflammatory, and pro-constrictive, all of which participate in the development of CIAKI. Shacham et al. and Liu Y et al. supposed that hs-CRP level were associated positively with the incidence of CI-AKI in patients with STEMI [29,34]. Su JZ et al. also showed that elevated levels of hs- CRP were related to the occurrence of CI-AKI in patients with ACS [35]. Consistent with previous studies, our study demonstrated that elevated levels of hs-CRP were independent factors for CI-AKI in patients undergoing elective percutaneous coronary angiogram.
Recently, the application of complex inflammatory indicators in the cardiovascular disorders has been progressively expanding. Compared with a single indicator, complex inflammatory indicators are more significant and stable. The main advantages are reflected in two points: 1) complex inflammatory indicators are less influenced by absolute number of a single index, and have predictive power in a larger range; 2) complex inflammatory indicators can integrate the two immune pathways of specificity and non-specificity, which is important prompt role in the outcome. Circulating lymphocytes are known to play an important role in specific immunity, while neutrophils, monocytes, platelets and the cytokines are believed to be associated with non-specific pathways. Studies showed that NLR was related to the incidence and mortality of CVD, including SAP, NSTEMI, and STEMI [36-41]. Kurtui A et al reported that lymphocyte counts in the CI-AKI group were significantly lower than the non-CI-AKI group among patients with NSTEMI [17]. Kaya A et al found that the higher levels of NLR were related to the increased risk of CI-AKI in STEMI patients [27]. High level of platelet count was an independent risk factor for CIAKI in patients with diabetes or renal insufficiency [42]. As the ratio of platelets to lymphocytes, PLR reflects overactive coagulation and inflammation, which may reduce renal blood flow and oxygen delivery [20]. Previous researches have proven that PLR had a strong predictive value for the occurrence of CI-AKI in patients with NSTEMI [37], and it was also an independent predictor of CIAKI in patients with ACS [20-24]. Similarly, another study among STEMI patients showed that the level of PLR in the CI-AKI group was significantly higher than that in the non-CI-AKI group [22,24], which also existed in the diabetes subgroup [23]. Domircelik and Kocas also found that the PLR level was an independent risk factor for predicting the occurrence of CI-AKI in patients with NSTEMI or ACS [20,21]. Monocytes and lymphocytes are important immune cells in the process of inflammation. MLR is a new inflammation indicator formed by combining monocytes and lymphocytes. Previous studies focused on the relationship between MLR and the prognosis of tumors, but few studies have explored the relationship between MLR and CI-AKI. Our study found that MLR also have a certain predictive effect on the incidence of CI-AKI in patients undergoing elective percutaneous coronary angiogram.
Although it is well known that elevated levels of inflammatory factors are associated with the incidence of CI-AKI in STEMI, the current widely used risk assessment models do not include complex inflammatory indicators such as NLR, MLR, and PLR [30,43,44]. Few studies have comprehensively explored the predictive value of applicable inflammatory factors for CI-AKI in patients undergoing elective percutaneous coronary angiogram, including NLR, MLR, PLR, hs-CRP and other indicators. At present, the treatment of CIAKI is rather limited, and the occurrence of CI-AKI will lead to prolonged hospitalization and poor prognosis. The main strategy to solve this problem is prevention. This study used large population cohort data to confirm the relationship between inflammatory factors and CI-AKI. Therefore, the predictive value of inflammatory factors levels should be emphasized in clinic to guide the prevention and treatment of CI-AKI.
This study has several limitations. First, as a multicenter, retrospective and observational study, the risk of bias cannot be completely ruled out, although we attempted to adjust for the confounding factors. Therefore, large-scale randomized controlled trials are needed to confirm our findings and hopefully develop new prevention strategies to reduce the incidence of CI-AKI. Second, our study conducted follow-up assessment of renal function within 1-3 days after PCI. Therefore, we might have missed a small part of population with the long-term increased Scr rather than deterioration of renal function within 72h, which lead to underestimate the incidence of CI-AKI.
Conclusion
Elevated levels of inflammatory factors, including hs-CRP, NLR, MLR, and PLR, are independent risk factors for the incidence of CI-AKI in patients undergoing elective percutaneous coronary angiogram.
References
- Caixeta A, Mehran R. Evidence-based management of patients undergoing PCI: Contrast-induced acute kidney injury. Catheterization and Cardiovascular Interventions. 2010; 75: S15-S20.
- Narula A, Mehran R, Weisz G, Dangas GD, Yu J, Généreux P, et al. Contrastinduced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. European heart journal. 2014; 35: 1533-1540.
- Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and Prognostic Importance of Acute Renal Failure After Percutaneous Coronary Intervention. Circulation: Journal of the American Heart Association. 2002; 105: 2259-2264.
- McCullough PA. Contrast-Induced Acute Kidney Injury. Journal of the American College of Cardiology. 2008; 51: 1419-1428.
- Pyxaras SA, Sinagra G, Mangiacapra F, Perkan A, Serafino LD, Vitrella G, et al. Contrast-induced nephropathy in patients undergoing primary percutaneous coronary intervention without acute left ventricular ejection fraction impairment. The American journal of cardiology. 2013; 111: 684-688.
- Liu ZZ, Viegas VU, Perlewitz A, Lai EY, Persson PB, Patzak A, et al. Iodinated contrast media differentially affect afferent and efferent arteriolar tone and reactivity in mice: a possible explanation for reduced glomerular filtration rate. Radiology. 2012; 265: 762-771.
- Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Medical Genomics. 2008; 2: 2-2.
- Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. Journal of the American College of Cardiology. 2010; 55: 2024- 2033.
- Sendeski MM. Pathophysiology of renal tissue damage by iodinated contrast media. Clinical and Experimental Pharmacology and Physiology. 2011; 38: 292-299.
- Guitterez NV, Diaz A, Timmis GC, O’Neill WW, Stevens MA, Sandberg KR, et al. Determinants of serum creatinine trajectory in acute contrast nephropathy. Journal of interventional cardiology. 2002; 15: 349-354.
- Fishbane S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clinical journal of the American Society of Nephrology: CJASN. 2008; 3: 281-287.
- Watabe H, Sato A, Hoshi T, Takeyasu N, Abe D, Akiyama D, et al. Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. International journal of cardiology. 2014; 174: 57-63.
- Crimi G, Leonardi S, Costa F, Ariotti S, Tebaldi M, Biscaglia S, et al. Incidence, prognostic impact, and optimal definition of contrast-induced acute kidney injury in consecutive patients with stable or unstable coronary artery disease undergoing percutaneous coronary intervention. insights from the all-comer PRODIGY trial. Catheterization and Cardiovascular Interventions. 2015; 86: E19-E27.
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske lekarske listy. 2001; 102: 5-14.
- Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk?. Journal of the American College of Cardiology. 2005; 45: 1638-1643.
- Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. The American journal of cardiology. 2006; 97: 993-996.
- Kurtul A, Yarlioglues M, Duran M, Murat SN. Association of Neutrophilto- lymphocyte Ratio with Contrast-induced Nephropathy in Patients with Non-ST-elevation Acute Coronary Syndrome Treated with Percutaneous Coronary Intervention. Heart, lung & circulation. 2016; 25: 683-690.
- Tokgoz S, Kayrak M, Akpinar Z, Seyithanoğlu A, Güney F, Yürüten B. Neutrophil lymphocyte ratio as a predictor of stroke. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013; 22: 1169-1174.
- Balta S, Ozturk C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. 2015; 26: 680- 681.
- Kocas C, Yildiz A, Abaci O, Karaca OS, Firdin N, Dalgic Y, et al. Platelet-to- Lymphocyte Ratio Predicts Contrast-Induced Nephropathy in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. Angiology. 2015; 66: 964-968.
- Demircelik MB, Kurtul A, Ocek H, Cakmak M, Ureyen C, Eryonucu B. Association between Platelet-to-Lymphocyte Ratio and Contrast-Induced Nephropathy in Patients Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndrome. Cardiorenal Medicine. 2015; 5: 96-104.
- Velibey Y, Oz A, Tanik O, Guvenc TS, Kalenderoglu K, Gumusdag A, et al. Platelet-to-Lymphocyte Ratio Predicts Contrast-Induced Acute Kidney Injury in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Angiology. 2017; 68: 419-427.
- Hudzik B, Szkodziński J, Korzonek-Szlacheta I, Wilczek K, Gierlotka M, Lekston A, et al. Platelet-to-lymphocyte ratio predicts contrast-induced acute kidney injury in diabetic patients with ST-elevation myocardial infarction. Biomarkers in medicine. 2017; 11: 847-856.
- Sun X, Li J, Zhu W, Li D, Chen H, Li H, et al. Platelet to Lymphocyte Ratio Predicts Contrast-Induced Nephropathy in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Angiology. 2018; 69: 71-78.
- Stacul F, Molen AJ, Reimer P, Webb JAW, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. European Radiology. 2011; 21: 2527-2541.
- Gao F, Zhou YJ, Zhu X, Wang ZJ, Yang SW, Shen H. C-Reactive Protein and the Risk of Contrast-Induced Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Intervention. American Journal of Nephrology. 2011; 34: 203-210.
- Kaya A, Kaya Y, Topçu S, Günaydin ZY, Kurt M, Tanboğa IH, et al. Neutrophilto- Lymphocyte Ratio Predicts Contrast-Induced Nephropathy in Patients Undergoing Primary Percutaneous Coronary Intervention. Angiology. 2014; 65: 51-56.
- Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation. 2010; 122: 2451-2455.
- Liu Y, Tan N, Zhou Y, Chen Y, Chen J, Chen J, et al. High-sensitivity C-reactive protein predicts contrast-induced nephropathy after primary percutaneous coronary intervention. Journal of nephrology. 2012; 25: 332-340.
- Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. Journal of the American College of Cardiology. 2004; 44: 1393-1399.
- Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. European heart journal. 2012; 33: 2007-2015.
- Akcay A, Nguyen Q, Edelstein CL. Mediators of Inflammation in Acute Kidney Injury. Mediators of Inflammation. 2009; 2009: 1-12.
- Fujii H, Li S, Szmitko PE, Fedak PWM, Verma S. C-Reactive Protein Alters Antioxidant Defenses and Promotes Apoptosis in Endothelial Progenitor Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006; 26: 2476- 2482.
- Shacham Y, Leshem-Rubinow E, Steinvil A, Keren G, Roth A, Arbel Y. High sensitive C-reactive protein and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention. Clinical and Experimental Nephrology. 2014; 19: 838-843.
- Su JZ, Xue Y, Cai WQ, Huang QY, Chai DJ, et al. Association between high sensitivity C-reactive protein and contrast induced acute kidney injury in patients with acute coronary syndrome undergoing percutaneous coronary intervention: impact of atorvastatin. Zhonghua Xin Xue Guan Bing Za Zhi. 2011; 39: 807-11.
- Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. The American journal of cardiology. 2010; 106: 470-476.
- Núñez J, Núñez E, Bodí V, Sanchis J, Miñana G, Mainar L, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. The American journal of cardiology. 2008; 101: 747-752.
- Park JJ, Jang H, Oh I, Yoon C, Suh J, Cho Y, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. The American journal of cardiology. 2013; 111: 636-642.
- Kurtul A, Murat SN, Yarlioglues M, Duran M, Celik IE, Kilic A, et al. Increased neutrophil-to-lymphocyte ratio predicts persistent coronary no-flow after wire insertion in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Clinics. 2015; 70: 34-40.
- Kaya A, Kurt M, Tanboga IH, Işık T, Günaydın ZY, Kaya Y, et al. Relation of Neutrophil to Lymphocyte Ratio With the Presence and Severity of Stable Coronary Artery Disease. Clinical and Applied Thrombosis/Hemostasis. 2014; 20: 473-477.
- Arbel Y, Shacham Y, Ziv-Baran T, Perl ML, Finkelstein A, Halkin A, et al. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long-term all-cause mortality in ST-elevation myocardial infarction patients. The Canadian journal of cardiology. 2014; 30: 1177-1182.
- Francuz P, Kowalczyk J, Swoboda R, Przybylska-Siedlecka K, Kozieł M, Podolecki T, et al. Platelet count and volume indices in patients with contrastinduced acute kidney injury and acute myocardial infarction treated invasively. Kardiologia polska. 2015; 73: 520-526.
- Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. The American journal of cardiology. 2004; 93: 1515-1519.
- Tziakas D, Chalikias G, Stakos D, Apostolakis S, Adina T, Kikas P, et al. Development of an easily applicable risk score model for contrast-induced nephropathy prediction after percutaneous coronary intervention: a novel approach tailored to current practice. International journal of cardiology. 2013; 163: 46-55.
