Abstract
Austenitic biomaterials are widely used in dentistry applications because of their biocompatibility, high corrosion resistance and appropriate mechanical properties. Despite their high resistance to uniform corrosion, they are prone to local pitting corrosion in halides containing environments. This paper concentrates on the effect of fluorides and chlorides containing mouthwash on corrosion resistance of austenitic surgical AISI 316L stainless-steel. Evaluation of the corrosion resistance is based on the results of exposure immersion tests (microscopic observation of attacked surfaces, mass losses of specimens) and on the results of the electrochemical potentio-dynamic polarization tests.
Keywords: Stainless Steel; Pitting Corrosion; Fluorides Containing Mouthwash; Exposure immersion Test; Potentiodynamic polarization Test
Introduction
Austenitic stainless steels are considered as attractive metallic biomaterials because of their biocompatibility, high corrosion resistance and appropriate mechanical properties. They are used in a variety of applications in dentistry (sterilized instruments, endodontic files in root canal therapy, metal posts in root canal treated teeth, temporary crowns, arch wires and brackets in orthodontics) [1,2,3].
Despite their high resistance to uniform corrosion, austenitic stainless steels are prone to local pitting corrosion in halides containing environments (especially chlorides and bromides). This corrosion form is typical for passivating metals and alloys. Aggressive ions present in solution can penetrate through the weakened places of surface passive film and cause its local breakdown and initiation of the pitting corrosion [4,5].
In contrast of corrosion resistance research in chloride containing environments [4,5], there have been few investigations into the effect of fluorides on passivity breakdown and pitting corrosion of stainless steels. Moreover, the results obtained are inconsistent and do not lead to the same conclusions [6-11].
AISI 316L is Cr-Ni-Mo low-carbon austenitic surgical stainless steel, which is widely used for orthodontic arch wires and brackets [1,2,8]. This paper concentrates on the effect of fluorides and chlorides containing mouthwash on corrosion resistance of this steel. Evaluation of the corrosion resistance is based on the results of exposure immersion tests (visual and microscopic observation of attacked surfaces, mass losses of specimens) and on the results of the electrochemical potentiodynamic polarization tests.
Materials and Methods
Experimental material
Cr-Ni-Mo austenitic stainless steel (Table 1) was used as an experimental material. The steel was purchased in sheet (1000x2000 mm) of 1.5 mm thickness and its treatment (marked as 2B) was based on annealing and pickling after smoothing rolling.
Content of Element [wt.%]
Cr
Ni
Mo
Mn
N
C
Si
P
S
Fe
16.79
10.14
2.03
0.82
0.05
0.02
0.31
0.03
0.001
Balance
Table 1: Chemical composition of experimental material.
Microstructure of experimental material (Figure 1, Figure 2) is created by polyedric austenitic grains with observable twins, which could be created by annealing or by rolling. Parallel lines arose by the rolling during the technologic process (Figure 2).

Figure 1: Microstructure of AISI 316L stainless steel, detail of austenitic
grains, cross section (Kalling’setch).

Figure 2: Microstructure of AISI 316L stainless steel, longitudinal section
(Kalling’setch).
Experimental methods and conditions
Colgate Plax Herbal Fresh mouthwash was used as an experimental solution for both immersion and potentiodynamic polarization tests. According to the data on the label it contains 0.05% sodium fluoride (Ingredients: Glycerin, Propylene glycol, Sorbitol, Poloxamer 407, Sodium saccharin, Cetylpyridinium chloride, Potassium sorbate, menthol, Citrus lemon peel oil, Camellia sinensis leaf extract). Used mouthwash was pH neutral, specific conductivity was1.121 mS.cm-1.
Exposure immersion tests: The specimen’s shape for immersion tests was rectangular (30 mm x80 mm x1.5 mm).The specimen surface was not treated (mechanically or chemically) only the edges were grinded by abrasive paper grain 600. The grease was removed by diethyl ether. The specimens were weighted out with accuracy ±0.000 01 g (Zilina, Slovakia, Mettler Toledo XS 205).Duration of immersion tests was 21 and 42 days (it means 504 and 1008 hours). It approximately simulated exposition during one year/ two years orthodontic treatment, respecting the recommendation to rinse the mouth two times a day (estimated time of mouthwash action for orthodontic appliance for half an hour every time). Ambient temperature during the experiment varied between 24 and 37°C (242/484 hours of total 504/1008 were at 37°C). The group of three parallel specimens was tested for both test durations (i.e. 3 specimens for 21 days test and 3 specimens for 42 days test) [5,12]. After exposure, the specimens were carefully brushed, washed by demineralized water, freely dried up and weighted out again.
Pitted surfaces of specimens after immersion tests were observed by the optical microscope (Zilina, Slovakia) NIKON AZ 100. Average corrosion rates (g.m-2.day-1) were calculated from corrosion losses (g.m-2) during the immersion tests.
Potentiodynamic polarization tests: Potentiodynamic polarization tests were carried out in a three electrode cell of corrosion measuring system (Volta Lab 10 with VSP unit). Anodic potentiodynamic polarization curves were obtained by the (Zilina, Slovakia) EC-LAB SOFT software. Potential between the sample and the electrolyte has been settled for 10 minutes before the polarization. Scan range was (-0.3V–1.2 V) vs. the open circuit potential and the scan rate was 1 mV/s. The surface of working electrode AISI 316Ti of 1 cm2 area was not mechanically treated, only rinsed with diethyl ether before measurement. The Saturated Calomel Electrode (SCE) was applied as the reference electrode and platinum foil as a counter electrode [5,12]. Tests were carried out at ambient temperature of 37±0.5 °C. At least five experiment repeats were carried out for tested specimen and the representative polarization curve was chosen.
Results and Discussion
Tested specimens were locally damaged by pitting during immersion test. The corrosion failure was not visible to the naked eye it was visualized at a microscopic observation. As can be seen (Figure 3, Figure 4), the pit’s shape is generally irregular, pit’s size varies.

Figure 3: Pitted surface of AISI 316L stainless steel after 21 days exposition
immersion test.
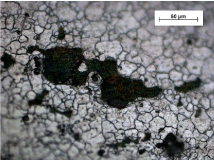
Figure 4: Pitted surface of AISI 316L stainless steel after 42 days exposition
immersion test.
Edges of corrosion pits seem to copy austenitic grains and it points to possibility of combination of two forms of corrosion attack, pitting and inter-granular corrosion [1,4,5]. There is no marked difference between pits appearance after 21 days and 42 days exposure (Figure 3, Figure 4). This observation correlates with fact, that mass losses of specimens after 21 days and 42 days exposure were quite similar. Average corrosion rates v [g.m-2day-1] calculated from these mass losses are shown and compared in (Figure 5). Average corrosion rate after 42 days is slightly lower than the rate after shorter exposure.
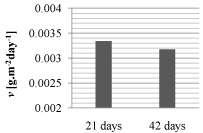
Figure 5: Average corrosion rates in dependence on exposure time.
Documented corrosion damage and mass losses indicate that nickel ions could be released from the biomaterial into the environment during exposure. Nickel ions are very harmful because they act allergenic, toxic and carcinogenic and can cause allergic contact dermatitis as well [2,8]. In vivo, Ni2+ ions may cross cell membranes using the Mg2+ ion transport system, and then bind to cytoplasmic ligands, although soluble Ni2+ is rapidly cleared. There is no specific mechanism for delivery of Ni2+ to target sites in the cell nucleus that may result in cancer causing genetic mutations. Released metal ions due to corrosion of the implanted steel can migrate into distant organs (liver, kidney, spleen), accumulate there and have harmful effects [2]. Not only nickel but also other steel alloying elements can cause damage within the human body environment. Large amounts of iron released from metallic implants can increase the level of iron in the blood. High blood levels of free ferrous iron react with peroxides and produce free radicals, which are highly reactive and can damage DNA, proteins, lipids. Iron typically damages cells in the heart and liver, which can cause significant adverse effects, including coma, liver failure and other long-term organ damage [2]. So corrosion phenomenon not only affects mechanical properties of the metal appliances, but also may influence the body due to leached metal ions [1,8,12].
Anodic potentiodynamic polarization curve expresses dependence of current density I [mA.cm-2] on potential E [V] in anodic direction. It enables determination of the passivity region and pitting potential Ep for the particular passivating material in the particular solution [1,4,5,12].
As can be considered from (Figure 6), passivity region of experimental material is broad, no breakdown of passivity, which indicates initiation of pitting corrosion, was recorded within it. Current density suddenly increases at the potential 1.014 V, which can be considered as transpassivation potential Et [14]. If potential is equal or higher than Et, surface passive film is dissolved, and active corrosion of material starts [1,4,5,12].

Figure 6: Anodic potentiodynamic curve for AISI 316L working electrode in
Colgate mouthwash.
Observed broad passivity region without any signs of pitting initiation does not correlate with results of immersion test. This difference might have been caused by changes in chemical composition of used mouthwash during exposition test because of leakage of volatile components (duration of potentiodynamic test was about half an hour, what was very short time in comparison with immersion test) and by temperature changes as well. It should be noted, that conditions of the immersion test were closer to real oral environment than conditions of the potentiodynamic polarization test.
However, more accurate results of exposure tests could be achieved if the temperature was constant (37°C) and the tests would be not continuous but interrupted, carried out always with a fresh dose of mouthwash.
Conclusions
Based on results of exposure immersion tests and potentiodynamic polarization tests can be concluded:
- Surface of austenitic biomaterial AISI 316L was attacked by local pitting corrosion during 21 and 42 days immersion test in Colgate mouthwash.
- Observed corrosion attack was reflected in mass losses during immersion test. Average corrosion rate calculated from mass losses after 42 days is slightly lower than rate after 21 days.
- Harmful nickel ions could be released from the biomaterial into the environment during exposure.
- Potentiodynamic polarization test indicated intact protective passive film on the surface of experimental material. It does not correlate with results of immersion test.
- Caution is recommended when using a fluoride containing mouthwash during long-term orthodontic treatment with apparatus made of austenitic biomaterial.
- Potentiodynamic tests are quick and convenient but not sufficient for an objective assessment of corrosion resistance. It is suitable to perform at least two independent tests before application of stainless steel into a real environment.
Acknowledgements
The research was supported partially by Scientific Grant Agency of Ministry of Education, Science and Sport of Slovak Republic and Slovak Academy of Science grant VEGA No. 1/0683/15 and by project KEGA No. 044ZU-4/2014.
References
- Palek P, Markoviová L, Zatkalíková V. Materiály pre biomedicínske ininierstvo (Materials for biomedical engineering). ilina: EDISilinskáuniverzita. 2015.
- Chen Q, Thouas GA. Metallic implant biomaterials. Materials Science and Engineering R. 2015; 87: 1-57.
- Omasta M, Hadzima B. Biodegradation properties of elektron 21 magnesium alloy coated by octa calcium phosphate. Manufacturing Technology. 2015; 15: 656-660.
- Szklarska-Smialovska Z. Pitting and crevice corrosion. Houston: Nace. 2005.
- Liptáková T. Bodová Korózia Nehrdzavejúcich Ocelí (Pitting corrosion of stainless steels). ilina: EDIS-ilinská Univerzita. 2009.
- Heravi F, Moayed MH, Mokhber M. Effect of Fluoride on Nickel-Titanium and Stainless Steel Orthodontic Arch-wires: An In-Vitro Study. Journal of Dentistry of Tehran University of Medical Sciences. 2015; 12: 49-59.
- Ghosh R, Singh RJ, Singh DDN. Role of fluoride in accelerating corrosion and pitting of steel in concrete environments. 2003; 56: 391-397.
- Shin JS, Oh KT, Huang CJ. In vitro surface corrosion stainless steel and NiTi orthodontic appliances. 2003; 19: 13-18.
- Carranza RM, Rodríguez MA, Bebak RB. Inhibition of chloride induced crevice corrosion in Alloy 22 by fluoride ions. Corrosion/2006 Conference and Exposition; 2006 March 12-16; San Diego, CA, USA.
- Li X, Wang J, Han EH, Ke W. Influence of fluoride and chloride on corrosion behaviour of NiTi orthodontic wires. Acta Biomater. 2007; 3: 807-815.
- Schiff N, Grosgogeat B, Lissac M, Dalard F. Influence of fluoridated mouthwashes on corrosion resistance of orthodontic wires. Biomaterials. 2004; 25: 4535-4542.
- Hadzima B, Liptáková T. Základy elektrochemickej korózie kovov (Fundamentals of electrochemical corrosion of metals), ilina: EDISilinskáuniverzita. 2010.
