Abstract
Background: The purpose of this study was to determine the impact of Chlor Hexidine diacetate salt (CHX) to the wettability of experimental resininfiltrants on smooth or rough glass surfaces.
Methods: Three experimental resin-infiltrants were produced: 1) TEGDMA Infiltrant (TI) + (0.5%camphorquinone + 1%DMAEMA and 0.1%BHT); 2) TI + 0.1% CHX; 3) TI + 0.2% CHX. TEGDMA was used as control. Wettability of experimental resin-infiltrants was assessed by sessile drop method for contact angle measurements with Digidrop (n = 12 per surface type). An image of each drop was captured and analyzed with the GBX Digidrop software. Data were subjected to two-way ANOVA followed by the Tukey’s test (a = 0.05).
Results: A significant interaction between materials and surface types was found (p<0.01). For smooth surfaces, there was no statistical difference among the materials (p>0.05). In contrast, the contact angleon rough surfaces was significantly reduced by the addition of 0.1% or 0.2% CHX (38±3° and 35±4° respectively) as compared to the control group (TEGDMA, 47±2° and TI 44±5°) (p<0.05).
Conclusion: The addition of CHX improved the wettability of experimental infiltrants on rough surfaces, regardless of the CHX concentration, suggesting that it might be an alternative approach for incipient enamel caries lesions.
Keywords: Resin infiltrant; Contact angle; Wettability; Chlorhexidine
Introduction
Although the prevalence of dental caries has declined remarkably in most industrialized countries over the recent years, population subgroups continue to experience a high incidence of dental caries [1]. Accordingly, it continues to be a large health issue for high-risk patients with approximately 70-96% of the children and adolescents presenting initial caries lesions, specifically in proximal surfaces [2,3]. In adults, up to 50% of patients show carious or restored proximal surfaces [3]. The lack of compliance with preventive behavior, e.g. good oral hygiene practices, eating habits and regular exposure to fluoride, has been reported as the major factor responsible for the prevalence of dental caries lesions in this group [4].
A promising non-drilling strategy to arrest and control proximal surface caries lesions has been extensively studied, which is typically referred as resin infiltration [5-10]. This strategy aims to occlude the porous structure of incipient enamel lesions by using low-viscosity light-curing resins mixtures. Resin infiltrates allow material to penetrate into the lesion body by promoting mechanical support in this fragile structure, reducing the enamel solubility and preventing the progression of the caries lesion [7]. Resin infiltration is a microinvasive approach to arrest and camouflage white spot lesions [8,10]. In this case, the wettability property becomes a critical factor, once the resin infiltrant covers and penetrates into the white spot lesion [5]. In order for infiltrants to be effective, they should shield and penetrate the enamel in problematic areas and have a relative low contact angle (wettability) [11].
Currently, the only commercially available infiltrant is Icon® (DMG, Hamburg, Germany), which has been described as a methacrylate-based resin matrix, initiators and additives by the manufacturer. Although a good performance to arrest initial caries lesions has been demonstrated, studies have shown that Icons’ properties must be improved. In a clinical trial study, Martignon et al. [12] (2012) showed the an increased efficacy of stabilization in the progression of proximal lesion by using Icon® as infiltrant (68%), with no statistical differences between Icon® and Prime Bond NT (60%) on white spot lesions. The rough tooth surface after the application of Icon® was questioned also, once the Icon® group exhibited an increased surface roughness even after polishing proceedings [13]. It is well known that a rough surface would increase biofilm accumulation, which can degrade the material surface, compromising resin durability and increasing staining and caries development [10,14].
It has been suggested that the addition of antibacterial agents, such as Chlor Hexidine diacetate (CHX) or digluconate in resin infiltrants may improve the ability of arresting incipient caries lesions and inhibit plaque accumulation on the surface of the material and surrounding dental tissue [15]. The hypothesis is that the addition of antimicrobial agents to resin infiltrants will result in a reduced biofilm growth in the infiltrated enamel because of their antibacterial properties. Such strategy seems highly attractive; especially considering that resin infiltrants are indicated for high caries risk patients [12].
Chlor Hexidine diacetate salt (CHX) is the most popular compound for antibacterial application in dental materials due to its wide spectrum of action [15,16]. It has been included in several classes of dental materials, such as glass-ionomer cements, resin-modified glass-ionomer cements, composites and adhesives improving and/ or extending the antimicrobial properties of these materials against cariogenic bacteria [15,17-19]. Other studies have confirmed the inhibition of bacterial growth on the tooth/restoration interface [20,21]. Furthermore, CHX can suppress the growth of Streptococcus mutans, and consequently, prevent dental caries development [16]. Therefore, the addition of CHX into the resin matrix is a promising approach to assure the releasing of CHX to local sites in the oral environment [15-17,19]. CHX is a symmetrical cationic molecule consisting of two 4-chlorophenyl rings and two biguanide groups connected by a central hexamethylene chain, which is considered a strong base and it is stable in the form of salts [22]. At low concentrations, small molecular weight substances, such as potassium and phosphorus, will leach out, exerting a bacteriostatic effect [22]. Nevertheless, in higher concentrations, CHX has bactericidal action due to precipitation or coagulation of bacteria’s cytoplasm, probably caused by protein cross-linking [22].
At concentrations of 0.1 and 0.2% Inagaki et al. 2013 [23] found that CHX did not impair the degree of conversion nor the Knoop hardness of experimental infiltrants based on TEGDMA (Triethylene Glycol Dimethacrylate), and that CHX-containing infiltrants presented antibacterial activity against Streptococcus mutans and Lactobacillus acidophilus [17]. Although, these findings were the first promising steps towards the characterization of CHX-containing resin infiltrants [15,17], further studies are required to access the potential of such association as a reliable strategy to deal with white spot lesions. In the current investigation, it was tested the hypothesis that the addition of CHX would affect the infiltrant wettability depending on whether the surface was rough or smooth.
Materials and Methods
Experimental design
The factors under analysis were
Materials: Neat monomer (TEGDMA=Triethylene Glycol Dimethacrylate-T); TEGDMA Infiltrant (TI) + [0.5% camphorquinone + 1% DMAEMA (2-Di-Methyl Amino Ethyl Meth- Acrylate) and 0.1% BHT (Butylated Hydroxy Toluene)]; TI + 0.1% CHX; TI + 0.2% CHX; and
Surface types: Smooth and rough. Twelve sessile drops were assigned to either smooth or rough (n = 12), and the wettability determined by the sessile drop method for contact angle measurements with Digidrop.
Experimental resin infiltrant preparation
In this study, three low viscosity resin infiltrants were prepared using the highly fluid dimethacrylate monomer TEGDMA (Sigma- Aldrich, St. Louis, USA). The photoinitiator system used in all infiltrants was 1.0 wt% DMAEMA and 0.5 wt% CQ (2-Dimethylaminoethyl Methacrylate and Camphoroquinone, Sigma-Aldrich, St. Louis, USA, respectively). The inhibitor BHT (Butylated Hydroxytoluene, Sigma-Aldrich, St. Louis, USA) was added at 0.1 wt% in order to prevent spontaneous initiation and propagation of the freeradical polymerization reaction [22]. The mentioned antibacterial/ antimicrobial agent CHX (Sigma-Aldrich, St. Louis, USA) was used at 0.1 and 0.2 wt%. In order to avoid premature polymerization, the resin components and blends were stored in dark glass opaque recipients at 4°C until use. The neat monomer TEGDMA was used as control group.
Evaluation of wettability-Contact angle
The surfaces used to evaluate the wettability of experimental infiltrants were on rough and smooth glass surfaces. In this way, both smooth and rough type microscope glass slides (Bioslide, Walnut, CA, USA) dimensions (25x76x1mm) were used with the rough surface being the frosted end of the slide. The smooth glass slide with a regular polished glass has a mean roughness (Ra) of 0.101μm and it was selected in order to evaluate the contact angle in an ideal situation for liquid spreading into the solid surface. The rough glass slide had mean roughness (Ra) of 0.553μm and it was selected to simulate the acid etching previously the infiltrant application.
Wettability of experimental resin infiltrant was evaluated by contact angle measurements [5]. The sessile drop method was performed using Digidrop GBX goniometer (Labometric Lda, Leiria, Portugal) with distinct glass surfaces (smooth and rough) (Figure 1). Briefly, each material was loaded into a 2mL syringe (insulin type) with a 22-gauge needle (Injex Ltda, S&aTilde;o Paulo, SP, Brazil) attached and coupled to the goniometer. Droplets (approximately 4μL) were applied onto the different glass surfaces. Twelve drops (n = 12) of each material were dispensed onto each of the glass surfaces. The measurement of contact angle was accomplished immediately after the infiltrant drop had formed on a glass slide (Figure 2). The test was accomplished at room temperature. Each drop’s corresponding image was captured without external light interferences. Images were frozen by PixeLink system (Barrington, IL, USA) and the measurements were made by the GBX Digidrop Windrop software (GBX Company, Bourg de Péage, France). The camera’s focus was adjusted in relation to the position of the table with glass slide surface and the needle tip for each image. The right and left angles were measured in degrees of the contact angle and average automatically calculated by GBX Digidrop software.
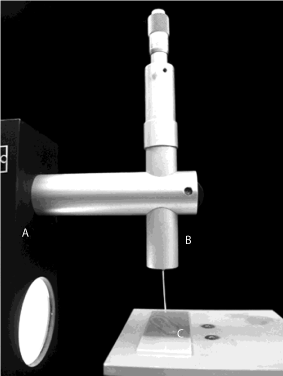
Figure 1: Experimental setup for contact angle measurement: A. Digidrop
GBX goniometer; B. Syringe with a 22-gauge needle; C. Glass surface.
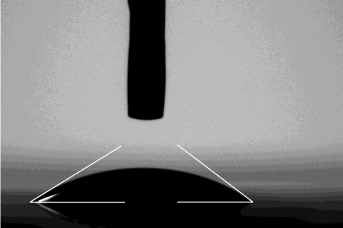
Figure 2: Photograph and measurements of a sessile drop on a GBX
Digidrop Windrop software. Right and left angles were measured in degrees
of the contact angle and average was automatically calculated.
Statistical analysis
Normal distribution of data were confirmed by the Lilliefors and Shapiro-Wilk statistical tests (a = 0.05). Data were submitted to the two-way ANOVA, considering Factor 1: material, in 4 levels: T, TI, TI + 0.1% CHX and TI + 0.2% CHX; Factor 2: surfaces, in 2 levels: smooth and rough and Tukey’s tests (a = 0.05). The software ASSISTAT 7.7 (DEAG-CTRN-UFCG, Campina Grande, PB, Brazil) was used to perform the statistical analysis.
Results
Table 1 shows mean values and standard deviation for the contact angles among the experimental groups. According to a twoway ANOVA, there was a significant interaction between Factor 1: material and Factor 2: surface (p<0.01). The lowest contact angle was found when CHX was added to experimental infiltrants, regardless CHX concentration (TI + 0.1% CHX and TI + 0.2% CHX) and surface type (smooth and rough). There were no significant differences among the experimental groups when smooth surface was considered (p>0.05), whereas the lowest contact angles were found on the rough surfaces, regardless CHX concentration (p<0.01).
Experimental Groups
Smooth Surface (S)
Rough Surface (R)
T
37.3±2.0 aB
47.0±1.8 aA
Ti
38.1±3.1 aB
43.6±4.5 bA
Ti + 0.1% CHX
37.6±1.7 aA
37.5±2.9 cA
Ti + 0.2% CHX
36.8±2.7 aA
35.2±3.7 cA
Different lowercase letters in column and uppercase letters in line show statistically significant differences according to Tukey’s test.
Table 1: Mean values ± Standard deviation for contact angle among the experimental groups.
Discussion
The contact angle assessment is widely used to characterize the wettability of resin materials [5,6,11,24-28]. Wettability is a critical factor for the adhesion of resin materials to the tooth surface as it defines how resin materials spread on tooth substrates. Contact angles lower than 90° indicate that the liquid has spontaneous capacity to wet the solid surface; the closer to 0°, the greater the surface energy and the wettability property between the interface solid/liquid [26]. In the current study, the hypothesis that the addition of different CHX concentrations would compromise the wettability of the infiltrant regardless of the surface type was rejected. In fact, the addition of CHX, regardless of its concentration, decreased the contact angle on rough surface, with no significant difference on the smooth surfaces.
Considering the experimental TEGDMA materials, regardless of the CHX addition, data analysis demonstrated that contact angles were lower than 90°, indicating that these materials present favorable spreading and good wettability, in homogeneous surfaces, such as glass slides. Glass surfaces were selected to accomplish the contact angle measurements because they are relatively inert and water free substrates, which contain no water unlike enamel and dentine [29]. The TEGDMA monomer usually exhibits hydrophilic properties because it contains polyethylene and glycols in the chemical chain. It is frequently used as a cross-linker in restorative resin materials because of its low viscosity characteristics enhancing polymerization reaction and wetting [24]. Kalachandra et al. 1993 [25] evaluated the contact angle of resin materials based on Bisphenol A Glycidyl Meth- Acrylate (BisGMA) in surfaces such as dentin, enamel, Poly-Methyl Meth-Acrylate (PMMA), and glass. They found decreased contact angles when the amount of TEGDMA was increased in monomer blends’, suggesting that the monomer should then increase the wettability of the resin materials [25]. Based on these findings, it is suggested to use the monomer in several monomer blends. However, when the different substrates were compared, contact angles increased according to the following sequence: glass<enamel<PMMA<dentin [25].
Paris et al. 2007 [5], evaluated the penetration coefficient of experimental resin blends based on BisGMA, UDMA (Urethane Di-Meth-Acrylate), TEGDMA, HEMA (Hydroxy Ethyl Meth- Acrylate) and ethanol. It was observed that resin blends containing high amounts of HEMA and TEGDMA presented low viscosity and high penetration coefficients in bovine enamel surface, with contact angles ranging from 3.2° to 33.3°. According to Li et al. 2011 [26], the penetration potential of a material depends on the surface tension of the liquid, and on the cosine of the angle formed between the liquid and contact surface, and on the dynamic viscosity of the fluid. Thus, an increased penetration capacity should be achieved with lower contact angles.
In the present study, data analysis revealed that surface type significant affected contact angles despite CHX concentration used. We speculate that smooth, flat, horizontal, chemically homogeneous and non-deformable substrates tend to form more balanced contact angle, with less variation of angles otherwise known as contact angle hysteresis [30]. In contrast, topographic heterogeneity, such as found for rough surfaces, may promote increased hysteresis [29], altering wettability properties of certain material because of air bubbles forming between the interface of the liquid and the solid surface [29]. Thereby, the air bubble being captured remains between the surface and the liquid, which may partially increase the contact angle [27]. However, when considering hydrophilic surfaces with isotropic and homogeneous characteristics, roughness can decrease the contact angle, due to the Wenzel theory, who mathematically demonstrated that when the contact angle is less than 90o, and liquid is added to a rough a surface, it became more hydrophilic. Nevertheless, this phenomenon was not a factor in the present investigation. Overall, our findings showed that a rough surface increased the contact angle for T and TI groups, but not for CHX groups.
In the current study, data analysis demonstrated that the presence of the photoinitiators particles and CHX, as compared to neat TEGDMA, significantly reduced the contact angles. In addition, it is important to note that contact angle reduction was more pronounced for the materials containing CHX, regardless its concentration, indicating a relation between material’s composition and altered cohesive forces within the liquid [29]. The addition of CHX powder particles may have reduced the cohesive force between the molecules of monomers, thereby reducing the surface tension of the blend. Analogously, it might be expected that the wettability of these materials may be improved in demineralized enamel.
Characterizing the dynamics of the molecules present in the liquid is a very important factor to allow a deeper understanding of its surface tension: molecules located within the volume of a liquid should have a resulting cohesion force equal to zero, as the surrounding molecules are distributed symmetrically around them [31]. However, the molecules located in free surface of the liquid are subject only to the forces of cohesion molecules in the layers just below the liquid contact surface. Thus, the surface acts as a “membrane” that tends to compress the liquid [31]. Considering the liquid materials evaluated by the current study, the presence of CHX and the light curing molecules increased the distance between the molecules of the TEGDMA and may have positively affected the wettability on rough surfaces, as it decreased the values of the contact angle. Also, considering that cationic molecules, such as CHX [16], have a tendency to increase water adsorption at the water-solid interface, decreasing the free energy and the superficial surface tension [28]. The materials used in this study did not contain water within their composition, the CHX molecules have cationic properties [15] and TEGDMA has more hydrophilic properties [32]; which suggests some interaction that cooperated to improve the wettability in the rough surfaces by the materials with an addition of the CHX. The same principle of reduction of cohesive forces between molecules [31] could be considered when the experimental infiltrant (TI) was compared with the control neat TEGDMA (T). The data suggest a significant reduction in contact angle within the rough surfaces, which is probably due to the addition of photo initiator system particles in TI. In addition, the experimental blends showed optimized performance on rough surfaces. In vitro studies should be considered to allow the characterization and refinement of the experimental materials properties that will serve as a basis for clinical studies in the future.
Conclusion
In conclusion, the presence of CHX in the experimental TEGDMA based infiltrants improved the wettability property of the materials on rough surfaces, regardless the concentration, which is important for infiltration on incipient enamel caries lesions.
Acknowledgement
The authors are grateful to the Department of Pediatric Dentistry, Piracicaba Dental School, State University of Campinas.Specifically, our thanks extend to Mr. Brian R. Morrow (University of Tennessee Health Science Center, Memphis, TN, USA) for critical reading and editing of the manuscript.The authors are also grateful toEspaço da Escrita– General Coordination of State University of Campinas for the language services provided. This research was supported by FAPESP – S&aTilde;o Paulo Research Support Foundation (grant # 2011/22149-0).
References
- Constante HM, Souza ML, Bastos JL, Peres MA. Trends in dental caries among Brazilian schoolchildren: 40 years of monitoring (1971-2011). Int Dent J. 2014; 64: 181-186.
- Peressini S, Leake JL, Mayhall JT, Maar M, Trudeau R. Prevalence of dental caries among 7- and 13-year-old First Nations children, District of Manitoulin, Ontario. J Can Dent Assoc. 2004; 70: 382.
- Kirkevang LL, Væth M, Wenzel A. Incidence of caries lesions in approximal surfaces: a radiographic study of a general adult Danish population. Caries Res.2011; 45: 538-546.
- Ashkenazi M, Bidoosi M, Levin L. Factors associated with reduced compliance of children to dental preventive measures. Odontology. 2012; 100: 241-248.
- Paris S, Meyer-Lueckel H, Cölfen H, Kielbassa AM. Penetration coefficients of commercially available and experimental composites intended to infiltrate enamel carious lesions. Dent Mater. 2007; 23: 742-748.
- Askar H, Lausch J, Dörfer CE, Meyer-Lueckel H, Paris S. Penetration of micro-filled infiltrant resins into artificial caries lesions. J Dent. 2015; 43: 832- 838.
- Kantovitz KR, Pascon FM, Nobre-dos-Santos M, Puppin-Rontani RM. Review of the effects of infiltrants and sealers on non-cavitated enamel lesions. Oral Health Prev Dent. 2010; 8: 295-305.
- Paris S, Schwendicke F, Keltsch J, Dörfer C, Meyer-Lueckel H. Masking of white spot lesions by resin infiltration in vitro. J Dent. 2013; 41: e28-34.
- Araújo GS, Sfalcin RA, Araújo TG, Alonso RC, Puppin-Rontani RM. Evaluation of polymerization characteristics and penetration into enamel caries lesions of experimental infiltrants. J Dent. 2013; 41: 1014-1019.
- Araújo GS, Naufel FS, Alonso RC, Lima DA, Puppin-Rontani RM. Influence of Staining Solution and Bleaching on Color Stability of Resin Used for Caries Infiltration. Oper Dent. 2015; 40: E250-256.
- Lv P, Liu Y, Wang Z, Liu S, Jiang L, Chen J, et al. In Situ Local Contact Angle Measurement in a CO2-Brine-Sand System Using Microfocused X-ray CT. Langmuir. 2017; 33: 3358-3366.
- Martignon S, Ekstrand KR, Gomez J, Lara JS, Cortes A. Infiltrating/sealing proximal caries lesions: a 3-year randomized clinical trial. J Dent Res. 2012; 91: 288-292.
- Yang F, Mueller J, Kielbassa AM. Surface Substance Loss of Subsurface Bovine Enamel Lesions After Different Steps of the Resinous Infiltration Technique: A 3D Topography Analysis. Odontology. 2012; 100: 172-180.
- Correr GM, Bruschi Alonso RC, Baratto-Filho F, Correr-Sobrinho L, Sinhoreti MA, Puppin-Rontani RM. In vitro long-term degradation of aesthetic restorative materials in food-simulating media. Acta Odontol Scand. 2012; 70: 101-108.
- Inagaki LT, Dainezi VB, Alonso RC, Paula AB, Garcia-Godoy F, Puppin- Rontani RM, et al. Evaluation of sorption/solubility, softening, flexural strength and elastic modulus of experimental resin blends with chlorhexidine. J Dent. 2016; 49: 40-45.
- Autio-Gold J. The role of chlorhexidine in caries prevention. Oper Dent. 2008; 33: 710-716.
- Inagaki LT, Alonso RC, Arau´jo GA, de Souza-Junior EJ, Anibal PC, Ho¨fling JF, et al. Effect of monomer blend and chlorhexidine-adding on physical, mechanical and biological properties of experimental infiltrants. Dent Mater. 2016; 32: e307-e313.
- Hiraishi N, Yiu CK, King NM, Tay FR, Pashley DH. Chlorhexidine release and water sorption characteristics of chlorhexidine-incorporated hydrophobic/ hydrophilic resins. Dent Mater. 2008; 24: 1391-1399.
- de Castilho AR, Duque C, Negrini TC, Sacono NT, de Paula AB, de Souza Costa CA, et al. In vitro and in vivo investigation of the biological and mechanical behaviour of resin-modified glass-ionomer cement containing chlorhexidine. J Dent. 2013; 41: 155-163.
- Tüzüner T, Kusgöz A, Er K, Tasdemir T, Buruk K, Kemer B. Antibacterial activity and physical properties of conventional glass-ionomer cements containing chlorhexidine diacetate/cetrimide mixtures. J Esthet Restor Dent. 2011; 23: 46-55.
- Korkmaz FM, Tüzüner T, Baygin O, Buruk CK, Durkan R, Bagis B. Antibacterial activity, surface roughness, flexural strength, and solubility of conventional luting cements containing chlorhexidine diacetate/cetrimide mixtures. J Prosthet Dent. 2013; 110: 107-115.
- Fardai O, Turnbull RS. A review of the literature on use of chlorhexidine in dentistry. J Am Dent Assoc. 1986; 112: 863-869.
- Inagaki LT, Alonso RCB, Anibal PC, Araújo GS, Souza EJ, Correr-Sobrinho L, et al. Antimicrobial and Polymerization Properties of Experimental Resin Blends ChlorhexidineAdded. J Dent Res. 2013.
- Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007; 28: 3757-3785.
- Kalachandra S, Taylor DF, Deporter CD, Grubbs HJ, McGrath JE. Polymeric materials for composite matrices in biological environments. Polymer. 1993; 34: 778-782.
- Li F, Li F, Wu D, Ma S, Gao J, Li Y, et al. The effect of an antibacterial monomer on the antibacterial activity and mechanical properties of a pit-andfissure sealant. J Am Dent Assoc. 2011; 142: 184-193.
- Morra M, Occhiello E, Garbassi F. Knowledge about polymer surfaces from contact angle measurements. Advances in Colloid and Interface Science. 1990; 32: 79-116.
- Gianino C. Measurement of surface tension by the dripping from a needle. Physics Education. 2006; 41: 440-444.
- Feitosa VP, Sauro S, Ogliari FA, Stansbury JW, Carpenter GH, Watson TF, et al. The role of spacer carbon chain in acidic functional monomers on the physicochemical properties of self-etch dental adhesives. J Dent. 2014; 42: 565-574.
- Long J, Hyder MN, Huang RY, Chen P. Thermodynamic modeling of contact angles on rough, heterogeneous surfaces. Adv Colloid Interface Sci. 2005; 118: 173-190.
- Nishioka M, Yamabe Y, Hisatsune K, Fujii H. Influence of Polishing of Denture Base Resin and Metal Surfaces on Wettability with Water and Saliva. Dent Mater J. 2006; 25: 161-165.
- Szymcyk K, Zdziennicka A, Janczuk B. Adsorption and wetting properties of cationic, anionic and nonionic surfactants in the glass-aqueous solution of surfactant-air system. Materials Chemistry and Physics. 2015; 162: 166-176.
