Abstract
The synthesis and maturation of collagen and extracellular matrix are adversely affected in diabetes. The aim of the present study was to evaluate the effect of periodontal inflammation on two basement membrane proteins with special reference to collagen IV, and laminin 5 and MMP-2 and MMP- 9 expression in gingival tissues of diabetic rats. The study was conducted on 22 male diabetes induced Wistar rats, with two groups: diabetes mellitus group and diabetes mellitus+periodontitis group. After sacrification on day seven, formalin fixed paraffin embedded gingival tissue specimens were immunohistochemically evaluated for expression of laminin 5, collagen IV, MMP-2, MMP-9. DM+periodontitis group had significantly higher values for MMP-9 expression in inflammatory cells. Higher MMP-2 staining were observed in epithelium of DM+periodontitis group (p<0.05). Positive correlations were observed between laminin 5 expression in gingival basal membrane and MMP-9 from inflammatory cells (rho=0.767, p=0.016). Positive correlations were also observed between inflammation score and MMP-9 expression from inflammatory cells (rho=0.617, p=0.043) in DM+periodontitis group. Periodontal inflammation plays a significant role in expression of laminin 5, MMP- 2, and MMP-9; contributing the collagenolytic balance in basal membranes of gingival epithelium in diabetes.
Keywords: Diabetes; Periodontitis; Matrix metalloproteinases; Collagen(s);
Introduction
Diabetes Mellitus (DM) is a complex metabolic disease characterized by a number of complications including periodontitis. The interrelationships between periodontitis and diabetes provide an example of systemic disease predisposing to oral infection, and once that infection is established, the oral infection exacerbates systemic disease [1].
Diabetes-induced changes in immune cell function produce an inflammatory immune cell phenotype (up regulation of proinflammatory cytokines from monocytes/polymorphonuclear leukocytes and down regulation of growth factors from macrophages). This predisposes to chronic inflammation, progressive tissue breakdown, and diminished tissue repair capacity. Another consequence of hyperglycemia is the alteration of circulating and immobilizing proteins. When proteins such as collagen or lipids are exposed to aldose sugars, they undergo non-enzymatic glycation and oxidation, resulting in the irreversible formation of Advanced Glycation End products (AGEs) [2,3]. These glucose-derived crosslinks contribute to reduced collagen solubility and turnover rate in diabetic animals and humans. Healing in diabetics involves decreased or impaired growth factor production [4], angiogenic response [5], keratinocyte and fibroblast migration and proliferation, collagen accumulation, and ECM components and their remodeling by Matrix Metallo Proteinases (MMPs) [6].
A potential mechanistic link between periodontal disease and diabetes involves the broad axis of inflammation, specifically the expression of pro-inflammatory cytokines and MMPs. The Basal Membrane (BM) and Extra Cellular Matrix (ECM) is the first and foremost barrier to protect the periodontal tissues from inflammation. MMPs, mainly generated by the host, play a major role in ECM breakdown [7]. The ECM of gingival tissues consists principally of collagens: type I, II, III, IV and non-collagen proteins such as laminin, elastin, fibronectin, tenascin, and proteoglycans are also present. Type IV collagen and laminin 5 are basic basal lamina components [8].
Vascular BM thickening is the foremost structural abnormality of diabetic micro-angiopathy. A close association between vascular BM thickening and the development of diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, and advanced periodontitis has been noted [9]. BM thickening develops from excess accumulation and reduced degradation of BM components.
In gingival epithelium, basal cells are the most metabolically active cells and are responsible for epithelial renewal and exchange of materials with the subepithelial tissues. The integrity of the ECM and BM is important in maintaining the stability of periodontal tissues [10-12]. Tissue integrity is maintained by a balance between matrix degradation and production, which is regulated to a large extent through the action of MMPs (particularly MMP-1, MMP-2, MMP-9) in both normal tissue remodeling and in pathological states [6,13]. Although the synthesis of vascular BM components are reported to be generally up-regulated by hyperglycemic conditions [14,15]; the effect of periodontal inflammation on epithelial BM turnover in diabetic gingival tissues is unknown. The alterations of BM proteins in the gingiva of diabetics with periodontitis or without periodontitis may be the earliest sign of changes in the vascular BMs of retina, and glomerulus. Hence, it may have value to evaluate the expression of BM components in gingival tissue in order to predict development of retinopathy.
The aim of the present study is therefore to analyze the expression of two basement membrane proteins (collagen IV and laminin 5) and two of their related MMPs (MMP-2 and MMP-9) in gingival tissues of diabetic rats and to evaluate whether possible alterations in expression patterns are related to the inflammation.
Materials and Methods
Animals
Twenty-two male healthy Wistar rats weighing 300-350g provided by Gazi University Medical Faculty Animal Experimentation Center were included for the study and all procedures were approved by the Animal Experimentation Committee of Gazi University. All animals were housed in separate cages at a constant temperature (24±2°C) in a 12-h light/dark cycle, and maintained on a standard laboratory diet.
Before test procedures, the body weights were recorded and peripheral venous blood was obtained from the tails in order to measure plasma glucose levels by the glycometer. Diabetes was induced with a single intraperitoneal injection of 50 mg/kg Streptozotocin (STZ) in citrate buffer solution (0.1 M pH 4.5). 10 days after the STZ injection, analysis of plasma glucose levels and body weights were repeated. DM induction was confirmed by the increase in the plasma glucose levels and body weight loss. Plasma glucose levels higher than 250mg/dl were accepted as diabetic. Twelve rats were utilized for the DM group (group 1) and twelve rats were utilized for DM+periodontitis (group 2). General anaesthesia was performed by intraperitoneal injection of ketalar (25mg/kg) for the surgical interventions. Experimental periodontitis was induced by ligature replacement around the maxillary first molar of the animals in the second group [16]. All rats received oral 1 ml carboxymetilcellulose daily for 7 days. On day 7, after sacrification, gingival specimens, 3x3 mm in dimension were obtained from the buccal sites of maxillary molar area. The specimens were prepared for histologic sectioning by fixation overnight in 10% paraformaldehyde. After fixation, and routine tissue processing the specimens were embedded in paraffin blocks.
Histopathological and immunohistochemical analyses
Three paraffin sections with 4-μm thickness were cut at the central region of each specimen. All sections were deparaffinized at 56°C and by xylene then incubated in absolute and 96% ethanol. The streptavidin-biotin method was used for immunohistochemical detection of MMP-2, MMP-9, Laminin 5 and Collagen IV expressions. The primary antibodies for Laminin 5 and Collagen IV are kindly provided by Prof. Koch, from Institute for Biochemistry II, Medical Faculty, Cologne, Germany.
MMP-2 and MMP-9: The sections were microwave treated in 0.01 M sodium citrate buffer (pH 6.0) for 10 minutes at 360W and final 5 min at 600W. After sections were rinsed with Phosphatebuffered saline (PBS, pH 7.6), the endogenous peroxidase activity was blocked by 1.5% hydrogen peroxide in distilled water for 15 minutes. After incubation overnight at 4°C with primary antibodies for MMP- 2 and MMP-9, a broad spectrum second antibody was applied for 20 min, followed by incubation in the HRP-streptavidin § for 30 min. Then, Di Amino Benzidine tetrahydrochloride (DAB) was used as chromogen for visualization of antibody. After counterstaining with Harris heamatoxylin, slides were dehydrated and mounted with mounting medium. Plasenta tissue was used as positive control tissue for MMP-2 and MMP-9 and for negative control slides instead of antibody only PBS was applied.
Laminin 5: The sections were microwave treated in 0.01 M sodium citrate buffer (pH 6.0) for 10 minutes at 360W and final 5 min at 600W. After sections were rinsed with Tris-Buffered Saline (TBS, pH 8.0), the endogenous peroxidase activity was blocked by 1.5% hydrogen peroxide in distilled water for 15 minutes. After incubation overnight at 4°C with Laminin 5 antibody at a 1:300 dilution in TBS, a broad spectrum second antibody was applied for 20 min, followed by incubation in the HRP-streptavidinfor 30 min. Then, Di Amino Benzidine tetrahydrochloride (DAB) was used as chromogen for visualization of antibody. After counterstaining with Mayer’s haematoxylin, slides were dehydrated and mounted with mounting medium. Breast tissue with infiltrative ductal carcinoma was used as positive control tissue for Laminin 5 and for negative control slides instead of antibody only TBS was applied.
Collagen IV: Sections were pre-treated with protease XXV (1mg/ ml in PBS buffer, pH 7.4) for 10 min at 37°C. After sections were rinsed with Phosphate-Buffered Saline (PBS, pH 7.6), the endogenous peroxidase activity was blocked by 1.5 % hydrogen peroxide in distilled water for 15 min. After incubation overnight at 4°C with Collagen IV antibody at a 1:100 dilution in PBS, a broad spectrum second antibody was applied for 20 min, followed by incubation in the HRP-streptavidin for 30 minutes. Then, DAB was used for visualization of antibody expression. After counterstaining with Mayer’s hematoxylin, slides were dehydrated and mounted. Skin tissue was used as positive control tissue. Negative control slides were treated with only PBS, no antibody was applied.
All slides were examined by two independent observers using Leica DM 4000 B light microscope with concordance. Inflammation was scored on a 4-grade scale, Grade 0-no inflammation, Grade 1- <15 cells/field, Grade 2 - 15-50 cells/field, and Grade 3 - >50 cells/ field, as described earlier [16]. Immunostaining density was scored on a scale of 0-3, where 0=no staining and 3=maximum staining. The pattern of the staining was evaluated as diffuse either focal. Scoring for Laminin 5 and collagen IV was performed for all basement membranes of gingival epithelium and blood vessels, and also for inflammatory cells. Scoring for MMP-2 and MMP-9 was performed for both basement membranes of gingival epithelium and blood vessels, gingival epithelial cells, fibroblasts, and inflammatory cells.
Statistical analysis
Paired sample t test was used to compare between baseline and post-STZ treatment for weight and blood glucose levels. Data were presented as mean and Standard Deviation (SD) values. Mann- Whitney U test was performed for comparison of Laminin 5, collagen IV, MMP-2 and MMP-9 in gingiva of both groups. Data were presented as median and Standard Deviation (SD) values. Spearman rank correlation test was done for the correlation of the inflammation score and BM and MMP protein expressions by a software package SSPS, v16.0 for Windows, IBM, Chicago, IL, USA.
Results
Clinical and histopathological analysis
As expected, significantly higher blood glucose levels and reduced body weights were detected for all animals in groups after inducing diabetes with STZ on day 7 (p<0.001) (Table I). The ligation successfully induced periodontal inflammation in the DM+P group and clinical signs of inflammatory responses (edema, redness, bleeding, fragility) were observed in all rats.
Blood glucose levels (mg/dl)
Weight (gr)
Baseline
10 days after STZ administration
Baseline
10 days after STZ administration
DM (n=11)
106.8±13.2
437.5±44.8*
244.6±42.3
212.3±36.3†
DM+P (n=11)
92.2±12.7
382.9±87.0*
254.0±33.3
230.0±29.1†
p<0.001
p<0.001
Significantly higher blood glucose levels and significantly lower weights in both groups compared to baseline (paired sample t test).
Table 1: Blood glucose and body weights of diabetic rats.
The biopsies involved marginal gingiva with oral gingival epithelium and gingival connective tissue from the maxillary molar sites which exhibited bone loss. The bone loss in ligature induced group was measured by the method described in elsewhere [16] (data not shown). Oral gingival epithelium showed hyperkeratosis and rete proliferation in both groups (DM; DM+P) whereas, acanthosis is slightly prominent in DM+P samples. The connective tissue of DM group demonstrated perivascular mild inflammatory infiltration. The DM+P specimens had moderate to severe degree of mononuclear inflammatory infiltration beneath the rete pegs with pronounced congestion in the capillaries (Figure 1A, B). The inflammatory infiltrate was mononuclear in nature with mainly plasma cell and lymphocyte content in both groups, however significantly higher inflammation scores were detected in DM+periodontitis group (Inflammation scores: 1.36, 2.72 in DM and DM+P groups respectively, p=0.008, Mann-Whitney U).
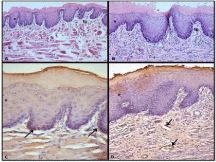
Figure 1: (A) Moderate keratosis in gingival epithelium (e) and mild
inflammation in lamina propria (lp) in DM group, original magnification x100
H&E, (B) Hyperkeratosis in acanthotic gingival epithelium (e) and severe
perivasculary inflammation in lamina propria (lp) in DM+P group, original
magnification x200 H&E, (C) Laminin expression in gingival BM in DM group,
original magnification x400, DAB, (D) Laminin immunoreactivity in vascular
basal membranes, original magnification x200, DAB.
Immunohistochemistry
Laminin 5 & Collagen IV: Laminin 5 expression was detected in both basement membranes of gingival epithelium and blood vessel walls in connective tissue of both DM (I) and DM+periodontitis groups. Inflammatory cells also showed immunoreactivity with laminin 5 (Figure 1 C,D).
Immunoreactivity for Type IV collagen was distinct in blood vessel basement membranes with diffuse staining pattern in gingival specimens of both groups.
MMP-2 and MMP-9: MMP-9 expression was detected in both gingival epithelium and lamina propria, in contrast MMP- 2 expression was mainly confined to the gingival epithelium. Both MMP-2 and MMP-9 were expressed weak to moderate in the spinous and granular layers of epithelium. The types of expressing cells were gingival epithelial cells and fibroblasts in lamina propria. Additively inflammatory cells showed positive immunoreactivity with MMP-9, and MMP-2 in both groups (Figure 2). DM+periodontitis group had significantly higher values for MMP-9 expression in inflammatory cells (Table 2, p=0.006, Mann-Whitney U). Higher MMP-2 staining were observed in epithelium of DM+periodontitis group (Table 2, p=0.004, Mann-Whitney U).
Gingival BM
Vascular BM
Inflammatory cells
DM (n=11)
DM+P (n=11)
DM (n=11)
DM+P (n=11)
DM (n=11)
DM+P (n=11)
Laminin 5
1,77±0,83
1,81±0,4
2,27±0,46
2,18±0,18
0
0
Collagen IV
0,63±0,67
0,50±0,52
0,81±0,75
1,00±0,44
0
0
MMP-2
3,89±3,38
4,10†±2,8
1,27±0,64
1,72±1,0
0,54±0,82
0,81±0,40
MMP-9
5,6±1,2
6,0±2,4
1,54±0,93
1,72±0,46
0,72±0,64
2,00±1,18†*
† Significantly higher values compared to DM group (p=0.006, Mann-Whitney U).
*Positively correlated with gingival BM Laminin 5 expression (rho=0,767, p=0,016) and inflammation score (rho=0,617, p=0,043) in DM+P group. No statistically significant differences at all other comparisions (p>0.05, Mann-Whitney U).
Table 2: Laminin 5, Collagen IV, MMP-2 & MMP-9 expression in gingival tissues of diabetic rats (mean ± SD).
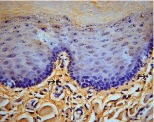
Figure 2: MMP-9 immunoreactivity in inflammatory cells in DM+P group,
original magnification x400, DAB.
Correlations
Positive correlations were detected between laminin 5 expression in basal membrane of epithelium and MMP-9 expressed in inflammatory cells (rho=0.767, p=0.016); and between inflammation score and MMP-9 expression from inflammatory cells (rho=0.617, p=0.043) in DM+periodontitis group (Figure 3).
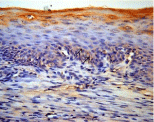
Figure 3: MMP-2 expression in gingival BM in DM+P group, original
magnification x200, DAB.
Discussion
Periodontal diseases and DM are closely related chronic diseases in which inflammation has crucial role in the development of both of them [1]. A bidirectional relationship between periodontitis and DM has been proposed. It is theorized that pro-inflammatory cytokines expressed by gingiva in periodontitis enter the systemic circulation leading the exacerbation of DM. Conversely, the elevated levels of the pro-inflammatory cytokines in DM may reach the gingiva leading to the aggravation of already existing periodontal disease [17,18]. Therefore, the study was focused on the gingival tissue of diabetic rats with or without periodontitis in order to evaluate the effect of inflammation on the tissue specimens with correlation of the morphology.
A major effect of high glucose conditions in vitro and in vivo is the modulation of patterns of integrin expression, including changes in the expression of MMPs and BM components which in turn contribute to the development of diabetic complications, primarily rethinopathy [19]. The formation of AGEs also occurs in gingival tissues with inflammation, and is found in high levels in those with diabetes. AGE modified collagen macromolecules accumulate in the tissues, and also resistant to degradation by MMPs, which are elevated in diabetic tissues, including the periodontium [6,20]. Various studies have reported that the prevalence and severity of non-oral diabetes related complications, including retinopathy, diabetic neuropathy, proteinuria and cardiovascular complications, are correlated with the severity of periodontitis [21-23]. However, the underlying inflammatory mechanisms responsible for the association of periodontal disease and increased risk of diabetic complications needs to be better clarified.
Taken all together, it can be enunciated that periodontal inflammation may play an important role in the BM turnover in diabetic gingival tissues. Moreover the correlation of MMPs with their related basement membrane proteins and inflammation may have input to current knowledge of its mechanism and clinical approach. Hence, the current study is designed to analyze collagen IV, laminin 5, MMP-2 and MMP-9 protein expressions in gingival tissues of diabetic rats in presence of periodontal inflammation.
It is inferred that the periodontal inflammated surface area can be used as a tool to accurately assess the periodontal inflamed tissue in a subject with periodontitis [24]. Since proinflammatory mediators could be poured in to the systemic circulation from the diseased gingival sites in severe periodontitis [25], gingival tissue may serve as a crucial biological material in the assessment of the inflammation degree and severity. Thus, the inflammation is evaluated in the gingival tissue of the diabetic rats with or without peridontitis in the present study. The results of the study showed significantly higher inflammation scores in DM+periodontitis group when compared to diabetics group. Our data is supported by the results of the other study, using type 2 diabetic animal model in the periodontitis group peaks at day 7 after removal of the ligation and then decreased gradually. This finding is in agreement with earlier reports demonstrating that both chronic diseases, in which inflammation plays central role in the pathogenesis, had a synergistic effect on each other with special reference to exuberated inflammatory infiltration of the gingival tissue [26].
In this study, using immunohistochemistry, we showed higher MMP-9 immune reactivity in inflammatory cells in gingival connective tissue in DM+P group (p=0.006) than DM group. Moreover we detected a positive correlation between MMP- 9 expression in inflammatory cells and laminin 5 expression in epithelial BM (rho=0.767, p=0.016). The present results indicate that inflammation might play an essential role on the expression of MMP- 9 and laminin 5. The data of a previous study which supports our findings, clearly demonstrated that laminin derived fragments play a role in the emigration of neutrophils and macrophages into sites of injury, as well as increased production of MMP-9 [27]. Macrophage migration peaks maximum level in gingiva at the fifth day of ligature which leads expression of MMP-9 gene. Subsequently, they become apparent in some cells of with intense cytoplasmic staining in the gingival connective tissue at 5-7 days after ligature, due to the accumulation of this enzyme inside the cells in later phases of gingival inflammation.
Immunohistochemistry for MMP-2 showed intensive cytoplasmic staining of the cells especially basal layer of the gingival epithelium and underlying connective tissue in DM+periodontitis group (p=0.004). Our data is in accord with the results of a previous study of rat gingivitis model, in which, MMP-2 was detected in gingival epithelial cells, with evident labeling of basal cell layer and in connective tissue as well. Moreover it was reported that MMP- 2 was more evident in the inflamed tissue between 5-7 days after ligature [27]. MMP-2 presented a continuous increase up to 7 days of inflammation and since macrophage recruitment increased MMP-2 content, higher expression of MMP-2 in the gingival epithelium of the DM+periodontitis group is the outcome of the inflammation.
Conclusion
We may speculate that periodontal inflammation, through stimulating laminin 5, MMP-2 and MMP-9 production released by the recruited inflammatory and resident cells may play an important role in the maintenance and over-expression of BM components in DM. Collecting together, and our results indicate that periodontal inflammation might play an important role in the turnover of gingival BM in diabetes. Future studies are needed to clarify if the elevated levels of MMP-9 and laminin 5 expression may be one of the underlying mechanisms, responsible for the association of periodontal disease and increased risk of diabetic complications.
References
- Gurav AN. Management of diabolical diabetes mellitus and periodontitis nexus: Are we doing enough? World J Diabetes. 2016; 7: 50-66.
- Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988; 318: 1315-1321.
- Ruderman NB, Williamson JR, Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992; 6: 2905-2914.
- Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004; 164: 1935-1947.
- Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005; 4: 3-8.
- Sorsa T, Tjaderhane L, Salo T. Matrix Metallo Proteinases (MMPs) in oral diseases. Oral Dis. 2004; 10: 311-318.
- Butler GS, Overall CM. Matrix metalloproteinase processing of signaling molecules to regulate inflammation. Periodontol. 2013; 63: 123-148.
- Jerdan JA, Michels RG, Glaser BM. Diabetic preretinal membranes. An immunohistochemical study. Arch Ophthalmol. 1986; 104: 286-290.
- Pinchback JS, Taylor BA, Gibbins JR, Hunter N. Microvascular angiopathy in advanced periodontal disease. J Pathol. 1996; 179: 204-209.
- Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol. 1997; 14: 33- 53.
- Bartold PM, Narayanan AS. Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol. 2006; 40: 29-49.
- Reynolds JJ, Meikle MC. The Functional Balance of Metalloproteinases and Inhibitors in Tissue Degradation: Relevance to Oral Pathologies. J R Coll Surg Edinb. 1997; 42: 154-60.
- Dong W, Xiang J, Li C, Cao Z, Huang Z. Increased expression of extracellular matrix metalloproteinase inducer is associated with matrix metalloproteinase-1 and -2 in gingival tissues from patients with periodontitis. J Periodontal Res. 2009; 44: 125-132.
- Gul N, Ozsoy N. The Ultrastructure of the Capillaries in the Gingiva of Alloxan- Induced Diabetic Rats. Cell Biochem Funct. 2003; 21: 311-315.
- Seppala B, Sorsa T, Ainamo J. Morphometric analysis of cellular and vascular changes in gingival connective tissue in long-term insulin-dependent diabetes. J Periodontol. 1997; 68: 1237-1245.
- Takada T, Yoshinari N, Sugiishi S, Kawase H, Yamane T, Noguchi T. Effect of restraint stress on the progression of experimental periodontitis in rats. J Periodontol. 2004; 75: 306-315.
- Sonnenschein SK, Meyle J. Local Inflammatory reactions in patients with diabetes and periodontitis. Periodontol. 2015; 69: 221-254.
- Franco C, Patricia HR, Timo S, Claudia B, Marcela H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int J Mol Sci. 2017.
- Kowluru RA, Mishra M. Regulation of Matrix Metalloproteinase in the Pathogenesis of Diabetic Retinopathy. Prog Mol Biol Transl Sci. 2017; 148: 67-85.
- Schmidt AM, Weidman E, Lalla E, Yan SD, Hori O, Cao R, et al. Advanced Glycation Endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res. 1996; 31: 508-515.
- Meenawat A, Punn K, Srivastava V, Meenawat AS, Dolas RS, Govila V. Periodontal disease and type I diabetes mellitus: Associations with glycemic control and complications. J Indian Soc Periodontol. 2013; 17: 597-600.
- Noma H, Sakamoto I, Mochizuki H, Tsukamoto H, Minamoto A, Funatsu H, et al. Relationship between periodontal disease and diabetic retinopathy. Diabetes Care. 2004; 27: 615.
- Banthia R, Raje S, Banthia P, Saral SK, Singh P, Gupta S. Evaluation of the association between periodontal disease and diabetic retinopathy. Gen Dent. 2014.
- Nesse W, Abbas F, van der Ploeg I, Spijkervet FK, Dijkstra PU, Vissink A. Periodontal inflamed surface area: quantifying inflammatory burden. J Clin Periodontol. 2008; 35: 668-673.
- Gurav AN. Periodontitis and Insulin Resistance: Casual or Causal Relationship? Diabetes Metab J. 2013; 36: 404-411.
- Chee B, Park B, Bartold PM. Periodontitis and type II diabetes: a two-way relationship. Int J Evid Based Healthc. 2013; 11: 317-329.
- Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, Miner JH, et al. A site on laminin alpha 5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J Immunol. 2003; 171: 398-406.
