Abstract
Aim: The purpose of this study was to examine whether fluoride may induce autophagy and explore the relationship between Beclin1, IRE1, Caspase-12 in fluoride-induced HAT-7 cells.
Methods: HAT-7 cells were cultured in different concentrations of fluoride for testing the appearance of autophagosomes by transmission electron microscope and Monodansylcadaverine (MDC) staining. Western blotting and RT-qPCR were carried out to examine the protein and mRNA expression of Beclin1, GRP78 and Caspase-12. Furthermore, correlation analysis was used to determine whether expression of autophagy and ER stress protein correlated in fluoride treated cells. Data were statistically analyzed by using correlation analysis and one-way analysis of variance.
Results: Our data suggest that excessive fluoride induces autophagy in HAT-7 cells, as the number of autophagosomes observed by Transmission Electron Microscopy (TEM) and Monodansylcadaverine (MDC) staining. There are difference among the four groups at protein and mRNA expression. Additionally, correlation analyses showed that Beclin1, GRP78 have a positive correlation with IRE1, suggesting that fluoride may induce autophagy via ERS pathway.
Conclusion: Our results suggest that IRE1, Beclin1 pathway may involved in fluoride induced autophagy in ameloblasts.
Keywords: Autophagy; Endoplasmic reticulum stress; Ameloblast; Fluoride; Toxicology; Apoptosis
Introduction
Dental fluorosis occurs as a result of high fluoride exposure during tooth formation. Fluorosed enamel is opaque white opaque with enamel pitting and a diminished enamel surface [1]. Ameloblasts are crucial for secreting and degrading enamel matrix proteins during enamel formation [2,3]. High levels of fluoride affect ameloblast physiology. Fluoride is a cellular stress inducer and causes Endoplasmic Reticulum (ER) stress in developing ameloblasts, which are disorganized arrangement, polarity defect, and whose Tomes’ process was shorten in dental fluorosis [4]. Excessive fluoride may result in apoptosis if not mitigated through internal defense systems including autophagy.
The ER is essential for the post-translational folding and alteration of membrane proteins and proteins destined for secretion [5]. ER function is sensitive to genetic mutations, physical or chemical stressors, and advanced age. Under these triggers, large amount of unfolded proteins accumulate and active an evolutionarily conserved adaptive process named the Unfolded Protein Response (UPR) which can rapidly induce expression of the ER chaperone protein, 78-kilodalton glucose-regulated protein (GRP78/BiP) and attenuate general protein synthesis [6]. If UPR failed to maintain intracellular homeostasis, the ERS can be induced. GRP78/BiP is the most abundant ER chaperone, and plays important roles in guiding protein folding and assembly, maintaining the permeability barrier of the ER during protein translocation, and degrading misfolded proteins. Importantly, GRP78/BiP also serves as a gatekeeper of the mammalian UPR [7]. The UPR is regulated by three transmembrane ER stress sensors: PKR-like ER Kinase (PERK), Inositol-Requiring Enzyme1 (IRE1), and Activating Transcription Factor 6 (ATF6). Under steady state conditions, PERK, IRE1, and ATF6 are bound and suppressed by GRP78. Under ER stress, these proteins are activated by dissociation with GRP78 [8].
IRE1, the primary sensor for unfolded proteins [9], is activated by trans-autophosphorylation, which causes splicing of the mRNA encoding the transcription factor X-box Binding Protein1 (XBP1) [10]. The IRE1 pathway is the most evolutionarily conserved UPR pathway and has a preapoptotic function that is involved in the activation of spliced XBP1 which can activate Beclin1 thus inducing Autophagy [11].
The UPR utilizes the ER-Associated Degradation (ERAD) pathway to direct misfolded proteins from the ER to the cytosol for degradation by the proteasome [12]. However, the ERAD pathway may not be sufficient to degrade severely misfolded proteins and protein aggregates. Autophagy is a more robust and likely degradation system for protein removal. Autophagy is an evolutionarily-conserved lysosomal-mediated bulk degradation system for intracellular components via fusion of an autophagosome containing dysfunctional cellular components with the lysosome [13,14]. Three distinct types of autophagy have been identified: macroautophagy, microautophagy, and chaperone-mediated autophagy. Macroautophagy (referred to as autophagy) is autophagy initiated by dissociating mTORC1 from Unc51-Like Kinase (ULK1) andATG13 complex which controls the formation of early autophagosomes. This triggers activation of the Beclin1 complex, composed of class 3 Phosphatidylinositol-3- Kinase3 (PI3KC3), vacuolar protein sorting 34 (Vps34), Beclin1, and other proteins required for nucleation and assembly of the doublemembraned phagophore.
Beclin1 is a BH3 domain protein that plays a significant role in autophagosome formation. In the PI3KC3 complex, Beclin-1 forms a platform that binds to several proteins that regulate the kinase activity of PIK3C3 to generate PI3P (phosphatidyl-inositol-3-phosphate). PI3P recruits Atg proteins and Double FYVE-Containing Protein 1 (DFCP1) to the site of autophagosome cradle formation. Beclin-1 stimulates autophagy after interacting with hVps. Beclin-1 can also suppress autophagy in complex with Bcl-2 [15]. Due to these antagonistic functions, Beclin-1 accumulation is considered a marker for Autophagy [14,16]. If autophagy induction fails to dampen the ER stress response, programmed cell death, apoptosis, occur.
Autophagy is an essential cytoprotective response to diseases such as ischemia, cancer, inflammation, and infection, but few studies have addressed how autophagy is regulated by ER stress in dental fluorosis [17]. Recent studies confirmed that excessive fluoride intake can induce autophagy as evidenced by the expression of autophagy related genes (Atg) and microtubule-associated protein 1 Light Chain 3 (LC3), a mammalian homolog of yeast Atg8 in LS8 cells line [18], but the connection of endoplasmic reticulum stress and autophagy in dental fluorosis has not been illustrated so far.
Based on these studies, we hypothesized that ER stress induced autophagy may contribute to the toxicity of dental fluorosis. In the present study, we examined how HAT-7 cells react to high dose fluoride exposure and found that autophagy is induced in response to fluoride toxicity. Our presented results suggested that fluoride may induce autophagy via the endoplasmic reticulum stress pathway.
Methods and Materials
Cell culture
Cell line: HAT-7 cell line was kindly provided by Iwate Medical University. HAT-7 cells are dental epithelial cells derived from the apical loop of rat incisors where is the dental stem cell niche. Moreover, HAT-7 cells have a potential to differentiate into ameloblasts [19].
Cells were grown in DMEM/F12 media supplemented with 10% FBS (Biological Industries, Israel) at 37°C with 5% CO2. Cells were divided into four groups and starved for 24 h in DMEM/F12 without FBS. Then the cells were treated with various concentrations of sodium fluoride (NaF) (China Pharmaceutical Group, China) (0mmol/L, 0.8mmol/L, 1.2mmol/L, or 1.6mmol/L) for 48 h for Western blot and RT-qPCR analysis.
Transmission Electron Microscope (TEM)
The ultrastructure of HAT-7 cells treated by NaF for 48 h (‘0 mmol/L, 1.2 mmol/L’ ) were analyzed by TEM. After fixation in 2% glutaraldehyde for 2 h, cells were dehydrated by alcohol gradient series before embedding in paraffin. The section was cut for 1μm by ultramicrotome (LKB-V, Sweden) and stained with uranyl acetate and lead citrate. Autophagosomes were examined and photographed by transmission electron microscopy (Hitachi 7650, Japan).
MDC staining
Cells were cultured on six well plates. At 48 h post-fluoride treatment, cells were washed with cold Phosphate Buffered Saline (PBS) twice. Cells were fixed in 4% paraformaldehyde for 30 min at 4°C. Autophagic vacuoles were labeled with 50 μL MDC (KeyGEN, China) for 1 h at 4°C in the dark. Cells were then washed twice with PBS and immediately analyzed by fluorescence microscopy using an inverted microscope equipped with a filter system (excitation filter: 355 nm, barrier filter: 512 nm) (Nikonte2000s, Japan). The images were obtained by a camera using the program NIS-Elements F 2.30.
RT-qPCR
Total RNA was extracted using TRIzol reagent (Life Technologies, USA). The concentrations of RNA were checked by the absorbance at 259 nm, and the RNA purity was determined by the OD 260/280 ratio (average >1.9). The samples were first reverse transcribed using the GoTaq® 1-Step RT-qPCR System (Promega, Madison, Wisconsin, USA). The mRNA expression levels of IRE1, GRP78, Beclin1, caspase 12, and actin were measured by RT-qPCR. The final volume of each sample (20 μL) contained 10 μL of GoTaq® qPCR Master Mix, 2 μL (100 ng) of forward and 2 μL (100 ng) of reverse primers (TaKaRa, Japan), 0.4 μL of GoScriptTM, and 4 μL (100 ng) of RNA. Primer sequences and annealing temperatures are as shown in Table1. The results were interpreted using the CT method. All RT-qPCR reactions were conducted at the following conditions: the reverse transcription program was as follows: 15 min at 42°C and10 min at 95°C. The templates were heated-denatured at 95°C for 10 s (40 cycles), followed by annealing at 72°C for 30 s (40 cycles), dissociation 15 s at 95°C, 15 s at 60°C, 15 s at 95°C, 15 s at 60°C.
Western blot
After treatment with NaF described above, HAT-7 cells were washed with ice-cold PBS and lysed in RIPA buffer [(50mM Tris pH 7.4, 150mM NaCl, 2mM EDTA, 0.1% SDS, 1% sodium deoxycholate, 1% Triton X-100) (Beyotime, China)] for 30min. Protein concentrations were determined by BCA reagent (Thermo, USA). 50μg of total protein lysate was separated by 10% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred to nitrocellulose filter membranes. The membranes were blocked in 5% skim milk for 1 hour and then incubated with antibodies: anti-IRE1 polyclonal antibody (1:500, Abcam, USA); anti-Beclin1 polyclonal antibody(1:1000, Abcam, USA); anti-GRP78 polyclonal antibody (1:1,000, Abcam, USA); anti-caspase12 polyclonal antibody (1:500), and anti-actin monoclonal antibody (1:1,000 Proteintech Group, USA) overnight at 4°C, respectively. After washing, membranes were incubated with a secondary detection antibody near infrared (DyLight, USA) conjugated goat anti-rabbit IgG (1:1,000, Abbkine, USA) in 5% skim milk for 1 h at room temperature, respectively. The bands were detected and quantified on an Odyssey CLx Infrared Imaging System (CLX, LI-COR, USA).
Statistical analysis
Statistic comparisons for experiments in cultured cells were performed using the one-way analysis of variance (ANOVA), performed by SPSS13.0 statistical software (SPSS, USA) and GraphPad Prism5. Statistical tests were two-sided with a significance level defined at p < 0.05. Data were expressed as mean ± standard.
Results
Fluoride regulates the expression of Beclin1 in HAT-7 cells
Autophagy is initiated by the formation of double membraned vesicles called autophagosomes. By visualization under the transmission electron microscope, in comparison to control group, double-membraned vesicles were more apparent in HAT- 7 cells incubated with NaF as shown in Figure 1. To further assess presence of autophagosomes, we probed for MDC, a marker for autophagicvacuoles. Following treatment with fluoride, punctate bright spots indicative of autophagic vesicles increased as shown in Figure 2. To make quantitative analysis, we also investigated expression of the autophagy related gene Beclin1. Gene expression analysis further supported that Beclin1 was upregulated in cells exposed to fluoride as shown in Figure 3 (p < 0.05). We performed a regression analysis to correlate fluoride treatment with Beclin1 expression and found that fluoride treatment correlated with Beclin1 expression in HAT-7 cells (Figure 3) (R= 0.599, p<0.05). HAT-7 cells were treated with the increasing concentrations of fluoride for 48h and protein lysate was extracted for Western blot analysis. As shown in Figure 3. Beclin1 protein expression increased significantly in a concentration dependent manner, and was highest at 1.2 mmol/L (p < 0.05). Therefore, these data demonstrate that fluoride exposure upregulates gene expression of Beclin1 leading to an accumulation at the protein level necessary for the autophagy process. Together with the microscopy, these data strongly suggest that fluoride exposure induces autophagy.
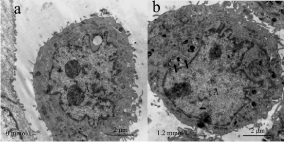
Figure 1: Effect of fluoride on autophagosomes.
Autophagosomes in HAT-7 cells were observed by transmission electron
microscopy. (a) HAT-7 cells in the control group (b) HAT-7 cells in fluoride
at 1.2 mmol/L. Autophagosomes were moch more apparent in experimental
group than control group. Autophagosomes in the tissues are indicated by
black arrows. A: cell nuclear. Scale bar: 2μm 29x14mm.
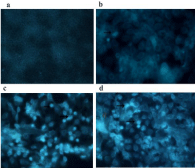
Figure 2: Effect of fluoride on autophagic vacuoles by MDC staining. (a)
0mmol/L (b) 0.8mmol/L (c) 1.2mmol/L (d) 1.6mmol/L. Punctate bright spots
indicative of autophagic vesicles increased in dose-dependent manner.
Magnification (a): 20×(b-d): 40×. Autophagosome in this tissue were referred
by arrows 31x31mm.
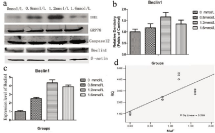
Figure 3: Effect of fluoride on the Beclin1 in HAT-7 cells.
HAT-7 cells were cultured in DMEM/F12 with different concentrations of NaF (0mmol/L; 0.8mmol/L; 1.2mmol/L; 1.6mol/L). The total RNA was extracted and
quantitative analysis of, Beclin1 (Figure 3c) was performed via RT-qPCR to measure the gene expression following exposure to varying concentration of fluoride.
Cells were treated with medium containing different concentrations of NaF. The protein expressions of Beclin1 was determined by western blot. The relative density
of Beclin-1 (Figure 3b) was represented as mean SD (n=3 each). Average relative RNA abundance of genes is represented as mean ± SD. Regression analysis
was performed and the results showed that fluoride does dependently induce the expression of the Beclin1 (Figure 3d). Results indicated the significant changes
(P < 0.05).
Fluoride induced endoplasmic reticulum stress in HAT-7 cells
To determine whether affected ameloblasts were under ER stress, we performed RT-qPCR and Western blot analysis of ER stress genes in cells after treatment with fluoride. GRP-78 has important roles in maintaining ER homeostasis, which can be bound to three transmembrane ER proteins in normal circumstance. RT-qPCR (R= 0.799, p < 0.05) and Western blot analysis (p < 0.05) showed elevated expression of GRP-78 in fluoride treated cells in a dose-dependent manner compared to the untreated control group (Figure 4a,b,c). IRE1 is one of the unfolded protein response transducers. To clarify whether fluoride induced ERS through IRE1 pathway, we examined the expression of IRE1 and XBP1 in HAT-7 cells. After treatment with fluoride, the expression of IRE1 in HAT-7 cells increased to the highest at 1.2mmol/L at both mRNA and protein levels (Figure 4d,e,f). The expression of XBP1 was significantly difference in different groups (Figure 4g).
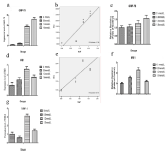
Figure 4: Effect of fluoride on the IRE1, GRP78 and XBP-1 in HAT-7 cells.
Cells were treated with medium containing different concentrations of NaF.
The protein and gene expression of IRE1 and GRP78 (Figure 4) were performed via RT-qPCR and Western Blot respectively, after exposure to varying concentration
of fluoride. Average relative RNA abundance of genes is represented as mean ± SD. Regression analysis was performed and the results showed that fluoride does
dependently induce the expression of the both genes. The gene expression of XBP-1 was performed by RT-PCT (Figure 4g).
Fluoride induced cell apoptosis via caspase-12 in HAT-7 cells
To determine whether apoptosis was induced by fluoride exposure, we investigated caspase-12 gene and protein expression. At 48h post-treatment, levels of caspase-12 were up-regulated in a dosedependent manner (Figure 5a,c) (p < 0.05). Likewise, a regression analysis showed that the expression of caspase-12 was induced by fluoride in dose-dependent manner as shown in Figure 5b (R= 0.645, p < 0.05). These data suggest fluoride induced apoptosis in HAT-7 cells through the caspase-12-dependent pathway.
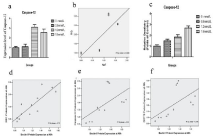
Figure 5: Figure 5a-c: The total RNA was extracted and quantitative analysis of Caspase-12 (Figure 5a) was performed via RT-qPCR to measure the gene
expression following exposure to varying concentration of fluoride. Cells were treated with medium containing different concentrations of NaF. The protein
expression of Beclin1 was determined by western blot. The relative density of Caspase-12 (Figure 5c) was represented as mean SD (n= 3 each). Regression
analysis was performed and the results showed that fluoride does dependently induce the expression of the Caspase-12 (Figure 3d). Results indicated the
significant changes (P < 0.05).
Figure 5d-f. Correlation analysis of Beclin1 with IRE1, GRP78, Caspase-12 in HAT-7 cells.
(d). Scatter plot of Beclin1 gene expression in cells versus IRE1 protein levels in cell culture medium after exposure to different concentrations of NaF at 48 h
(correlation analysis P < 0.05). (e). Scatter plot of Beclin1 gene expression in cells versus caspase-12 protein levels in cell culture medium after exposure to
different concentrations of NaF at 48 h (correlation analysis P < 0.05). (f). Scatter plot of Beclin1 gene expression in cells versus GRP78 protein levels in cell culture
medium after exposure to different concentrations of NaF at 48 h (correlation analysis P < 0.05). The trendline is shown for each graph as well as the value of the
coefficient of determination (R-sq liner).
Correlation Analysis of IRE1, GRP78, Caspase-12 with Beclin1 protein expression
To determine whether expression of autophagy and ER stress protein correlated in fluoride treated cells, we performed Pearson’s correlation analysis. Our results suggested expression of Beclin1 proteins strongly correlated with IRE1, GRP-78, and caspase-12 at 48h as shown in Figure 5d-f (R= 0.73, p<0.05; R= 0.344, p<0.05; R= 0.34, p < 0.05, respectively).
Discussion
ER stress is a cellular response to the accumulation of misfolded and unfolded proteins. Cells respond to ER stress via a protective mechanism termed the Unfolded Protein Response (UPR) to repair normal ER function [20]. In line with this idea, our current results demonstrate that HAT-7 cells suffered from severe ER stress induced by fluoride. The levels of GRP78, a key UPR modulator, were increased in HAT-7 cells exposed to fluoride for 48h. Under stress, misfolded proteins induce GRP78 dissociation three membrane proteins PERK, IRE1 and ATF6, causing activation of the UPR. These results suggest UPR was activated due to ERS.
Autophagy is responsible for degrading cytoplasmic macromolecules, protein structures and damaged organelles [21]. The most specific marker of autophagy is the appearance of double membrane-enclosed vesicles termed autophagosomes. After maturation and fusion with lysosomes, thereby forming autolysosomes, cytosolic contents are degraded. Earlier studies reported that excess formation of autophagosomes was accompanied by increasing membrane biosynthetic activity [22]. In our study, formation of autophagosomes in fluoride-treated cells increased significantly, indicating that autophagy may occur in dental fluorosis. However, to maintain the integrity of the whole cell, we failed to detect the double-membraned structure in low resolution. A significant increase of the MDC labeling (marker for late autophagy vacuoles) was recorded with the concentration increasing. To investigate autophagy in ameloblasts further, we characterized the autophagy gene, Beclin1, in fluoride-treated cells. Autophagy-initiating PI3K/ Vps34 kinase complex generates Phosphatidylinositol 3-Phosphate (PI3P) which is essential for recruitment of subsequent ATG proteins on the initial autophagosome membrane. Beclin1 interacts with Vps34 and is required for the formation of the complex that activates autophagy. Our results suggested that Beclin1 was upregulated due to fluoride exposure in HAT-7 cells (p < 0.05).
The ERS response and autophagy processes are tightly connected in Drosophila and mammals. Our data show the expression of IRE1 and Beclin1 were enhanced by fluoride treatement in a dose dependent manner. Furthermore, correlation analysis showed that Beclin1 expression strongly correlates with IRE1 (R= 0.73, p < 0.05). In mammals, three ER-stress sensor proteins (IRE1, PERK, ATF6) react to the accumulation of unfolded proteins, but IRE1 and PERK have demonstrated roles in the ER stress response [23]. Regulation of the ER stress response by IRE1 occurs through two methods. IRE1 has RNase activity (required for XBP1 mRNA splicing) which causes a transcription factor to induce chaperone protein production and increase UPR gene expression involved in ERAD. Conversely, IRE1- TRAF2 complex formation activates pro-apoptotic JNK signaling through interaction with a different subset of pro-apoptotic proteins at ER membrane. Previous studies reported that IRE1-medinated activation of JNK leads to the activation of autophagy through XBP1 splicing and activation of Beclin1 [24,25].
Alternatively, Deegan et al. reported that PERK is a better modulator of autophagy using pharmacological inhibitors of PERK and IRE1 in HCT119 colon cancer cells. Beclin1 contains a BH3 domain that can be bound and inhibited by Bcl-2 or Bcl-XL proteins which can suppress autophagy and apoptosis [26]. As previously demonstrated, IRE1 activated by reticular BH3-only effector pathways does not splice XBP1. Other regulators in the UPR pathway such as ATF6 may regulate XBP1 instead [27].
Based on the previous studies, we speculated that fluoride causes ER stress and triggers autophagy to sustain cell viability through the ER stress pathway. IRE1 and Beclin1 play an important role in the regulation of this pathway. However, Mie Ito found that accumulation of intracisternal granules cause ER damage, activating autophagy without the UPR. These data suggest that the ER stress-dependent autophagy activation differs from the UPR [28]. IRE1 and PERK are required for this process, but whether they operate independently or in collaboration remain unclear. Further studies are required to fully elucidate the mechanisms regulating IRE1 signal transduction.
As we know, some of the publication has reported the relationship between ERS and fluoride [29,30]. Likewise, Wei et al have found that Autophagy may protect MC3T3-E1 cells from fluoride-induced apoptosis. Our previous results showed that fluoride induced apoptosis at 1.6mmol/L by flow cytometry. In this paper, the expression of Beclin1 and caspase-12 were up regulated by Western blot and RT-qPCR. Beclin1 expression was strongest at 1.2mmol/L, but decreased at 1.6mmol/L. Caspase-12 expression was highest at 1.6mmol/L. The fluoride on apoptotic occurrence was not paralleled by a similar interactive increase in autophagy. These results suggest that autophagy observed in ameloblasts may be a protective escape mechanism form apoptoic cell death at this time point. A previous study showed that there are two pathways of ERAssociated Death (ERAD): 1) by activating the CCAAT/Enhancer- Binding Protein (C/EBP) homologous protein (CHOP/GADD153) or by2) caspase-12 [31]. Caspase-12 is located in the ER [32]. Of the eleven number of capspases, only caspase-12 is specifically activated by stimuli inducing ER stress [33]. Our results suggest apoptosis occurs at fluoride concentrations of 1.6mmol/L. Under these conditions, efforts at autophagy cease. Depending on external factors, autophagy is thought as an alternative cell death pathway. Autophagy and apoptosis are important cellular pathways regulated by Bcl-2 phosphorylation [34]. Bcl-2 negatively regulates Beclin-1-dependent autophagy, and also exerts an anti-apoptotic effect on other members of the Bcl-2 family. Therefore, the expression of Beclin1 may be a crucial controller between apoptosis and autophagy [35,36]. Based on our results, we hypothesize that autophagy may be involved in the fluoride-induced apoptosis mediated by the endoplasmic reticulum stress pathway.
Acknowledgement
We thank Iwate Medical University for generously providing us with ameloblast-lineage cells.
References
- Bronckers AL, Lyaruu DM, DenBesten PK. The impact of fluoride on ameloblasts and the mechanisms of enamel fluorosis. J Dent Res. 2009; 88: 877-893.
- Lacruz RS, Smith CE, Chen YB, Hubbard MJ, Hacia JG, Paine ML. Geneexpression analysis of early- and late-maturation-stage rat enamel organ. Eur J Oral Sci. 2011; 119: 149-157.
- Reith EJ, Cotty VF. The absorptive activity of ameloblasts during the maturation of enamel. Anat Rec. 1967; 157: 577-587.
- Wei W, Gao Y, Wang C, Zhao L, Sun D. Excessive fluoride induces endoplasmic reticulum stress and interferes enamel proteinases secretion. Environ Toxicol. 2013; 28: 332-341.
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011; 475: 324-332.
- Feng J, Chen X, Sun X, Wang F, Sun X. Expression of endoplasmic reticulum stress markers GRP78 and CHOP induced by oxidative stress in blue light-mediated damage of A2E-containing retinal pigment epithelium cells. Ophthalmic Res. 2014; 52: 224-233.
- Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004; 4: 966-977.
- Tsang KY, Chan D, Bateman JF, Cheah KS. In vivo cellular adaptation to ER stress: survival strategies with double-edged consequences. J Cell Sci. 2010; 123: 2145-2154.
- Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005; 102: 18773-18784.
- Zou X, Xu J, Yao S, Li J, Yang Y, Yang L. Endoplasmic reticulum stressmediated autophagy protects against lipopolysaccharide-induced apoptosis in HL-1 cardiomyocytes. Exp Physiol. 2014; 99: 1348-1358.
- Liu H, Zeng Q, Cui Y, Zhao L, Zhang L, Fu G, et al. The role of the IRE1 pathway in excessive iodide- and/or fluoride-induced apoptosis in Nthy-ori 3-1 cells in vitro. Toxicol Lett. 2014; 224: 341-348.
- Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006; 4: e423.
- Deegan S, Saveljeva S, Gorman AM, Samali A. Stress-induced selfcannibalism: on the regulation of autophagy by endoplasmic reticulum stress. Cell Mol Life Sci. 2013; 70: 2425-2441.
- Halder P, Datta C, Kumar R, Sharma AK, Basu J, Kundu M. The secreted antigen, HP0175, of Helicobacter pylori links the unfolded protein response (UPR) to autophagy in gastric epithelial cells. Cell Microbiol. 2015; 17: 714- 729.
- Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl- 2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2011; 18: 60-71.
- Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007; 282: 4702-4710.
- Deegan S, Saveljeva S, Gupta S, MacDonald DC, Samali A. ER stress responses in the absence of apoptosome: a comparative study in CASP9 proficient vs deficient mouse embryonic fibroblasts. Biochem Biophys Res Commun. 2014; 451: 367-373.
- Suzuki M, Bartlett JD. Sirtuin1 and autophagy protect cells from fluorideinduced cell stress. Biochim Biophys Acta. 2014; 1842: 245-255.
- Kawano S, Morotomi T, Toyono T, Nakamura N, Uchida T, Ohishi M, et al. Establishment of dental epithelial cell line (HAT-7) and the cell differentiation dependent on Notch signaling pathway. Connect Tissue Res. 2002; 43: 409- 412.
- Celli J, Tsolis RM. Bacteria, the endoplasmic reticulum and the unfolded protein response: friends or foes? Nat Rev Microbiol. 2015; 13: 71-82.
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005; 12: 1542-1552.
- Matsuo S, Nakagawa H, Kiyomiya K, Kurebe M. Fluoride-induced ultrastructural changes in exocrine pancreas cells of rats: fluoride disrupts the export of zymogens from the rough endoplasmic reticulum (rER). Arch Toxicol. 2000; 73: 611-617.
- Mori K. Frame switch splicing and regulated intramembrane proteolysis: key words to understand the unfolded protein response. Traffic. 2003; 4: 519-528.
- Castillo K, Rojas-Rivera D, Lisbona F, Caballero B, Nassif M, Court FA, et al. BAX inhibitor-1 regulates autophagy by controlling the IRE1alpha branch of the unfolded protein response. EMBO J. 2011; 30: 4465-4478.
- Margariti A, Li H, Chen T, Martin D, Vizcay-Barrena G, Alam S, et al. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem. 2013; 288: 859-872.
- Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008; 27: S137-148.
- Klee M, Pallauf K, Alcala S, Fleischer A, Pimentel-Muinos FX. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009; 28: 1757-1768.
- Ito M, Nakagawa H, Okada T, Miyazaki S, Matsuo S. ER-stress caused by accumulated intracistanal granules activates autophagy through a different signal pathway from unfolded protein response in exocrine pancreas cells of rats exposed to fluoride. Arch Toxicol. 2009; 83: 151-159.
- Kubota K, Lee DH, Tsuchiya M, Young CS, Everett ET, Martinez-Mier EA, et al. Fluoride induces endoplasmic reticulum stress in ameloblasts responsible for dental enamel formation. J Biol Chem. 2005; 280: 23194-23202.
- Sharma R, Tsuchiya M, Bartlett JD. Fluoride induces endoplasmic reticulum stress and inhibits protein synthesis and secretion. Environ Health Perspect. 2008; 116: 1142-1146.
- Cao Y, Hao Y, Li H, Liu Q, Gao F, Liu W, et al. Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int J Mol Med. 2014; 33: 809-816.
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006; 7: 880-885.
- Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000; 150: 887- 894.
- Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009; 284: 2719-2728.
- Ku B, Woo JS, Liang C, Lee KH, Jung JU, Oh BH. An insight into the mechanistic role of Beclin 1 and its inhibition by prosurvival Bcl-2 family proteins. Autophagy. 2008; 4: 519-520.
- Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy. 2008; 4: 947-948.
