Introduction
Matrix metalloproteinases, produced by both infiltrating and resident cells of the periodontium, play a role in physiological (such as tooth eruption) and pathological (such as periodontitis) events [1]. The evidence for the role of matrix metalloproteinases in periodontal destruction has accumulated for over three decades. It has been shown that an imbalance between activated matrix metalloproteinases and their host-derived endogenous inhibitors leads to pathological breakdown of the extracellular matrix during periodontitis and numerous other diseases [2].
The matrix metalloproteinases are an important family of zincand calcium-dependent endopeptidases secreted or released by a variety of host cells that function at neutral pH and utilize the various constituents of the extracellular matrix as their substrates [3]. Their main function is to catalyze the breakdown of proteins in the cell plasma membrane or within the extracellular matrix [4].
These proteinases are involved in a number of physiological events such as embryonic development, involution of the post-partum uterus, tissue remodeling, salivary gland morphogenesis and tooth eruption, in addition to various pathological processes such as (but not limited to) periodontal disease, arthritis, cancer, atherosclerosis, diabetes, pulmonary emphysema and osteoporosis [5].
Periodontal tissue cells including fibroblasts, keratinocytes, neutrophils, macrophages and endothelial cells constitute the primary source of MMPs.
Matrix Metalloproteinase Activity and Periodontal Disease
In periodontal diseases, matrix metalloproteinases play key roles in the degradation of the extracellular matrix, basement membrane and protective serpins as well as in the modification of cytokine action and activation of osteoclasts.
The extracellular matrix not only consists of collagen fibrils but also their associated proteoglycans and fibronectin, which must be removed first in order for the collagenase to have access to the collagen substrate. Matrix metalloproteinase-3 (stromelysin) is effective at degrading proteoglycans and fibronectin (Figure 1).
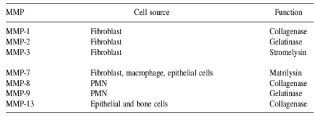
Figure 1: MMPs produced by periodontal cells [4].
It is important to note that a large number of vertebrate cells produce these matrix metalloproteinases. Both resident gingival and periodontal ligament fibroblasts produce collagenases that are thought to be involved in normal tissue turnover. Inflammatory cells such as neutrophils and macrophages produce matrix metalloproteinases, with neutrophils being the major source of collagenase and gelatinase in inflammatory diseases such as periodontitis. Epithelial cells can also produce elevated levels of these enzymes, which may facilitate the apical migration and lateral extension of the junctional epithelium and the subsequent loss of connective tissue attachment [5].
The evidence for the role of matrix metalloproteinases in periodontal destruction is strong and has been supported over many years by a number of findings, including the production of elevated levels of collagenase by diseased gingival tissues in culture, the detection of elevated levels of active rather than latent collagenase in the fluid of the periodontal pocket and in extracts of the adjacent inflamed gingival tissue, and the presence of matrix metalloproteinase messenger RNA in cells of the periodontal lesion, such as periodontal ligament and gingival fibroblasts as well as keratinocytes, endothelial cells, osteoblasts and even osteoclasts [6].
Additional evidence for this pathogenic pathway is the presence of elevated matrix metalloproteinase protein in periodontal lesions supported by immunohistochemical studies [6]. Moreover, the ability of matrix metalloproteinase inhibitors, such as doxycycline, to retard periodontal breakdown in humans and experimental animals, further supports the pathological role of these proteinases.
Sources of matrix metalloproteinase production regulated by cytokines and growth factors
Each of the major cell types in the periodontal tissues (polymorphonuclear leukocytes, fibroblasts, keratinocytes, macrophages and endothelial cells) is capable, when activated by various cytokines, arachidonic acid metabolites and growth factors, of producing a battery of different matrix metalloproteinases [2]. In addition, bone cells such as osteoblasts (stimulated by parathyroid hormone, vitamin D3, IL-1, TNF-a, prostaglandin E2 and endotoxin) and osteoclasts, can express and synthesize matrix metalloproteinases.
Recently, neutral proteinases such, as matrix metalloproteinase-9 (92-kDa gelatinase, also called gelatinase-B) were also found to be secreted by human osteoclasts in vitro [7] and expressed by rabbit metacarpal bones in vivo as demonstrated by in situ hybridization, suggesting that other matrix metalloproteinases may also be produced by osteoclasts during bone collagen degradation [8].
Matrix metalloproteinase- 13 (collagenase-3) has been associated with collagen degradation during bone resorption mediated by osteoclasts.
A membrane-type matrix metalloproteinase (MT1-matrix metalloproteinase or matrix metalloproteinase-14) expressed by osteoclasts may activate pro-matrix metalloproteinase- 13 during the proteolytic cascade responsible for the breakdown of the organic matrix during bone resorption [4].
Temporal relationship of gingival matrix metalloproteinase activity and alveolar bone loss
Numerous studies have detected collagenase and gelatinase in gingival crevicular fluid and inflamed gingiva and have demonstrated positive correlations between the activities of these matrix metalloproteinases and the severity of periodontal disease based on cross-sectional studies [9,10]. Matrix metalloproteinase-8 was found to be the main interstitial collagenase in gingival extracts and gingival crevicular fluid. Matrix metalloproteinase-13 (collagenase-3) has been identified in the GCF of patients with periodontitis.
Matrix Metalloproteinase Inhibitors
The role of inhibitors is particularly important because it is an imbalance between the activated matrix metalloproteinases and their endogenous inhibitors that leads to pathological breakdown of the extracellular matrix in diseases [11,12].
This rationale has led to the development of a number of synthetic inhibitors, matrix metalloproteinase inhibitors, not only as ‘‘tools’’ in the study of the mechanisms involved in matrix metalloproteinase– associated pathology, but also as potential therapeutic agents.
This can be accomplished with the use of drugs that can:
• Inhibit the synthesis and/or release of these enzymes
• Block the activation of precursor (latent) forms of these matrix metalloproteinases (pro-matrix metalloproteinases)
• Inhibit the activity of mature matrix metalloproteinases
• Stimulate the synthesis of endogenous tissue inhibitors of matrix metalloproteinases
• Protect the host’s endogenous inhibitors from proteolytic inactivation
These inhibitors can be –endogenous or exogenous.
Endogenous inhibitors
Endogenous or natural inhibitors such as tissue inhibitors of matrix metalloproteinases and a2-macroglobulin bind in a noncovalent fashion to members of the matrix metalloproteinase family. Tissue Inhibitors of Matrix Metalloproteinases (TIMPs) probably control matrix metalloproteinase activities peri-cellularly, whereas a2- macroglobulin functions as a regulator of matrix metalloproteinases in body fluids [4]. MMP and TIMP levels in GCF change during periodontitis. MMP-8 in GCF may be suitable as a marker to monitor periodontal health. This shows that several options are available for the diagnostic and therapeutic use of MMPs and TIMPs, but also that more research is required, especially with respect to the role of other matrix proteinases.
Exogenous (synthetic) inhibitors
• Zn2+ and Ca2+ chelating agents [13]
EDTA and 1, 10- phenanthroline are potent inhibitors of enzyme activity in vitro, but they are toxic and not used in vivo as therapeutic agents
• Multiple synthetic peptides [14]
Multiple synthetic peptides have been formulated in an attempt to synthesize more specific chelators including phosphorus containing peptides, sulfur-based inhibitors and peptidyl hydroxamic acid derivatives.
• Phosphorus containing peptides [14]
These are potent inhibitors of metalloproteinases produced by the substitution of a tetrahedral phosphorus atom for the carbonyl carbon atom in a peptide substrate.
• Sulfur based inhibitors of the matrix metalloproteinases [15]
These were prepared by replacing the scissile CO-NH bond of the peptide with various sulfur-containing functional groups. The mercaptan derivatives were the most potent inhibitors of collagenases, gelatinases and stromelysin compared with all other sulfur- based inhibitors of matrix metalloproteinases in vitro.
• Hydroxamic acid derivatives [16]
The most widely used synthetic peptides, and the ones receiving the most attention as potential pharmaceutical agents, are the hydroxamic acid derivatives (Galardin, Batimastat). These are prepared by adding a hydroxamic acid residue at the C-terminus of the peptide as a metal-chelating moiety.
• Bisphosphonates [17]
Most recently bisphosphonates, primarily designed to modulate osteoclast function and not specifically designed to be matrix metalloproteinase inhibitors, have been found to inhibit matrix metalloproteinases 1, 3, 8 and 13 in vitro; the mechanism of action may involve cation chelation.
• Tetracycline and its analogues [5]
Subantimicrobial Dose of Doxycycline (SDD) is a 20 mg dose of doxycycline (Periostat) that is approved and indicated as an adjunct to SRP in the treatment of chronic periodontitis. It is taken twice daily for 3 months. The dose exerts its therapeutic effect by enzyme, cytokine and osteoclast inhibition rather than by any antibiotic effect. At present, SDD is the only HMT specifically indicated for the treatment of chronic periodontitis that is approved by U.S. Food and Drug Administration (FDA) and accepted by the ADA.
Chemically Modified Tetracyclines (CMT) 18 are one of the most promising groups of HMTs. These non-antibiotic tetracycline analogues are tetracycline molecules that have been modified to remove all antibiotic properties, but which retain host modulatory, anti-collagenolytic effects. The CMTs are also designed to be potent inhibitors of proinflammatory mediators and can increase levels of anti-inflammatory mediators such as IL-10. CMTs are also being studied for other effects such as, inhibition of tumour cell invasion and attenuation of intimal thickening after arterial injury. CMTs will likely emerge as drugs that have beneficial effects in a variety of disease states because of their host modulation capabilities.
Isothiazolone
The isothiazolones can inhibit cartilage proteoglycan degradation without decreasing synthesis. These drugs appear to target the activation step of matrix metalloproteinase without being active against the active enzyme.
Phospholipase A2 inhibitors
There has been some work on the use of inhibitors of phospholipase A2, which controls the rate-limiting step in prostaglandin production [19].
Cranberry fraction
In a study using human cells stimulated by A. actinobacillus the lipopolysaccharide-induced MMP-3 and MMP-9 responses of fibroblasts and macrophages were inhibited in a dose-dependent manner by the cranberry fraction. This fraction was found to inhibit fibroblast intracellular signaling proteins, a phenomenon that may lead to a down-regulation of activating protein-1 activity. MMP-3, MMP-9 and elastase activities were also efficiently inhibited by the cranberry fraction, even when it was used at low concentrations. These results suggest that cranberry compounds offer promising perspectives for the development of novel host-modulating strategies for an adjunctive treatment of periodontitis [20,21].
A study was conducted to investigate the effect of non-dialyzable material (NDM) prepared from cranberry juice concentrate on the proteolytic activities of P. gingivalis, T. forsythia and T. denticola. The effect of NDM on gingipain and Dipeptidyl Peptidase IV (DPP IV) activities of P. gingivalis, trypsin-like activity of T. forsythia and chymotrypsin-like activity of T. denticola was evaluated using synthetic chromogenic peptides. NDM dose-dependently inhibited the proteinases of P. gingivalis, T. forsythia and T. denticola as well as type I collagen and transferrin degradation by P. gingivalis [22]. Although a number of matrix metalloproteinase inhibitors have been developed over the past decade, only a few have been found to be safe and effective, particularly after oral administration The tetracycline analogues are currently the only matrix metalloproteinase inhibitors approved by the US Food and Drug Administration being used clinically.
Matrix Metalloproteinase Inhibition by Tetracycline Analogues: Multiple Mechanisms?
The initial demonstration that tetracycline antibiotics can inhibit host-derived matrix metalloproteinases, and by a mechanism independent of the antimicrobial properties of the drugs, was made in germ-free rats with experimentally induced diabetes, a model of in vivo excess collagenase activity [23].
The first mechanism proposed was the ability of tetracyclines to inhibit already active matrix metalloproteinases (collagenase and gelatinase) in the extracellular matrix, a mechanism found to be associated with the Zn2+ or Ca2+ binding properties of the tetracycline molecule [24].
This proposed mechanism has been supported by the following observations:
• Adding excess Ca2+ (mM concentrations) or excess Zn2+ (mM concentrations) eliminated the ability of the TC analogues to inhibit collagenase activity in vitro.
• Structural evaluation of collagenase has revealed that the enzyme contains a secondary Zn2+ , outside the active site of the enzyme (in addition to an active site Zn2¹ ), which in addition to a secondary Ca2¹ , helps maintain the conformation and catalytic activity of the enzyme.
• Tetracyclines such as doxycycline block the matrix metalloproteinases in vitro apparently by non-competitive inhibition.
These findings suggest that the tetracyclines may bind to the secondary Zn2+ (and to a lesser extent, Ca2+) in collagenase, thus altering the conformation of the enzyme molecule and blocking its catalytic activity in the extracellular matrix.
Additional inhibitory mechanisms of these drugs include their ability to prevent the conversion of pro-matrix metalloproteinases in the extracellular matrix into active matrix metalloproteinases [4].
Two different mechanisms of doxycycline inhibition of recombinant human pro-matrix metalloproteinase- 8 have been detected [25].
Based on Western blot analysis of the different molecular species of matrix metalloproteinase-8, it is proposed that, during activation, doxycycline binds to the pro-matrix metalloproteinase (complexing with Ca2+), thus altering the enzyme’s conformation and resulting in excessive degradation of the proteinase to small enzymatically inactive fragments.
At the US National Cancer Institute (National Institutes of Health), Stetler-Stevenson et al. (personal communication) recently examined the interaction kinetics of gelatinase A with either doxycycline or chemically modified tetracycline-3 and found that the former (commercially available) tetracycline analogue functioned as an uncompetitive inhibitor, whereas the latter exhibited a mixed mechanism of inhibition and was more potent.
In addition, structural features in the hemopexin-like domain of the matrix metalloproteinases may modify the response of the matrix metalloproteinases to doxycycline and differences within the catalytic domain of the matrix metalloproteinases may also contribute to their susceptibility to tetracycline inhibition (such as of the collagenases, matrix metalloproteinase-13 and matrix metalloproteinase-8 appear to have a wider catalytic cleft and are more sensitive to tetracycline inhibition than matrix metalloproteinase-1).
Scavenging of polymorphonuclear leukocytes-generated reactive oxygen metabolites (such as HOCl) by tetracyclines may prevent the oxidative conversion of pro-matrix metalloproteinases in the extracellular matrix into active matrix metalloproteinases, and this property of tetracyclines appears not to depend on the metal-ion binding properties of these drugs.
Tetracyclines appear to inhibit extracellular matrix breakdown by indirect mechanisms as well. In this regard, the serum protein a1- antitrypsin (also called a1-proteinase inhibitor) is the host’s major defense against another family of tissue-destructive proteinases, the serine proteinases (particularly polymorphonuclear leukocyte elastase). Matrix metalloproteinases are now known to degrade and inactivate a1- antitrypsin, so that tetracycline-inhibition of the matrix metalloproteinases could protect elastase susceptible substrates (such as elastic fibers, fibronectin, and proteoglycans and tissue inhibitors of matrix metalloproteinases) from proteolytic attacks well.
Another potential indirect mechanism by which the tetracyclines may inhibit extracellular matrix breakdown could be through inhibition of activation of pro-tumor necrosis factor-a, hereby leading to a decrease in the formation of the powerful cytokine, tumor necrosis factor-a [26].
Mechanisms of Inhibition of Connective Tissue Breakdown by Tetracycline’s
Mediated by extracellular mechanisms
• Direct inhibition of active MMPs – dependant on Ca++ and Zn++ binding properties of tetracyclines.
• Inhibition of oxidative activation of pro MMPs independent of cation binding properties of tetracyclines.
• Tetracyclines disrupt activation by promoting excessive proteolysis or pro-matrix metalloproteinases into enzymatically inactive fragments – dependant on cation binding properties of tetracyclines.
• Inhibition of MMPs protects a1 – proteinase inhibitor, thus indirectly decreasing serine proteinase (such as PMNL elastase) activity.
Mediated by cellular regulation
• Tetracyclines decrease cytokines, inducible nitric oxide synthase, phospholipase A2, prostaglandin synthase
• Effects on protein kinase C, calmodulin
Mediated by pro-anabolic effects
• Tetracyclines increase collagen production
• Tetracyclines increase osteoblast activity and bone formation
Exogenous Matrix Metalloproteinase Inhibitors and Their Proposed Role in the Treatment of Periodontitis
Treatment of chronic periodontitis with medications and down-regulators (such as doxycycline, chemically modified nonantimicrobial tetracycline-derivates, bisphosphonates, and their combinations) and therapeutic applications of anticollagenase drugs (such as synthetic matrix metalloproteinase inhibitors) in endotoxininduced tissue-destructive periodontitis model in rats have been shown to result in reduced levels of matrix metalloproteinase-8 and other matrix metalloproteinase and in activity that is associated with clinically beneficial outcomes [27].
Other advantages for periodontal treatment are that tetracyclines, particularly doxycycline, tend to be highly concentrated in the gingival crevicular fluid at levels 5-10 times greater than those found in serum and these antibiotics show substantivity because they bind to the tooth structure and are slowly released as still-active agents [5]. The ability of tetracyclines and doxycycline, in particular, to inhibit MMP activity was first identified in the early 1980s [4]. The only matrix metalloproteinase inhibitors which have been tested for the treatment of periodontitis are members of the tetracycline family of compounds. Because the chemically modified tetracyclines are not yet approved for human use, all the following references to clinical trials involve the use of commercially available tetracyclines and their semi-synthetic analogues, minocycline and, most importantly, doxycycline [5]. The tetracycline antibiotics have been found to inhibit host-derived collagenases and other matrix metalloproteinases by a mechanism independent of the antimicrobial activity of these drugs; this effect may suppress connective tissue breakdown during periodontal disease.
Two therapeutic strategies based on the host-modulating properties of tetracyclines are currently being developed:
• The use of low-dose doxycycline (the most potent anticollagenase of commercially available tetracyclines) formulations, which do not appear to result in tetracycline side effects such as the emergence of antibiotic-resistant microorganisms; and
• The production of a family of chemically modified tetracyclines that have lost their antimicrobial activity, but have retained their anticollagenase activity [28].
Conclusion
From this review it can be concluded that MMPs are the important family of endopeptidases capable of degrading ECM and basement membrane leading to the periodontal diseases. These MMPs are mainly responsible for the degradation of collagen fibers. High levels of activity are found mainly in gingival crevicular fluid in inflammatory conditions like periodontitis, cancer etc. Therefore, detection and reduction of these levels are important for inhibiting the progressive lesions. By keeping all this in mind the therapeutic interventions should focus on reducing the levels of activity MMPs.
References
- Birkedal-Hansen H, Moore W, Bodden M, Windsor L, Birkedal- Hansen B, De Carlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993; 4: 197-250.
- Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal disease. J Periodontol. 1993; 64: 474-484.
- Genco RJ, Slots J. Host Responses in Periodontal Diseases. J Dent Res. 1984; 63: 441-451.
- Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005; 32: 108-129.
- Ryan ME, Golub LM. Modulation of matrix Metalloproteinase activities in Periodontitis as a treatment Strategy. Periodontology. 2000; 24: 226-238.
- Hansen BH. Role of matrix metalloproteinases in human periodontal disease. J Periodontol. 1993; 64: 474- 484.
- Wucherpfennig A, Li YP, Stetler-Stevenson W, Rosenberg A, Stashenko P. Expression of 92 kD Type IV collagenase/gelatinase B in human osteoclasts. J Bone Miner Res. 1994: 9: 549-556.
- Tezuka KI, Nemoto K, Tezuka Y, Sato T, Ikeda Y, Kobori M, et al. Identification of matrix metalloproteinase 9 in rabbit osteoclasts. J Biol Chem. 1994; 269: 15006-15009.
- Hansen BH. Role of matrix metalloproteinases in human periodontal disease. J Periodontol. 1993; 64: 474- 484.
- Overall C, McCulloch C, Sodek J. Identification of polymorphonuclear leukocyte collagenase and gelatinase activity in mouthrinse samples: correlations with periodontal disease activity in adult and juvenile periodontitis. J Periodontal Res. 1990; 25: 257-267.
- Golub L, Evans R, McNamara T, Lee H, Ramamurthy N. A non-antimicrobial tetracycline inhibits gingival matrix metalloproteinases and bone loss in Porphyromonas gingivalis-induced periodontitis in rats. Ann N Y Acad Sci. 1994; 732: 96-111.
- Ryan M, Ramamurthy N, Golub L. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr Opin Periodontol. 1996; 3: 85-96.
- Greenwald R. Tetracyclines may have potential benefit in rheumatoid arthritis but not for the reasons you think. J Clin Rheumatol. 1995; 1: 185-189.
- Galardy R, Grobelny D, Kortylewicz Z, Poncz L. Inhibition of human skin fibroblast collagenase by phosphorus-containing peptides. Matrix. 1992; 259- 262.
- Schwartz M, Venkataraman S, Libby A, Mookhtiar K, Mallya S, Van Wart H, et al. Sulfur-based inihibitors for matrix metalloproteinases. Matrix. 1992; 309-310.
- Nagai Y, Hattori S, Odake S, Okayama T, Obata M, Morikawa T. Preparation of peptidyl hydroxamic acid derivatives which inhibit interstitial collagenases. Matrix. 1992; 1: 313.
- Teronen O, Konttinen Y, Lindqvist C, Salo T, Ingman T, Lauhio A, et al. Inhibition of matrix metalloproteinase-1 by dichloromethylene bisphosphonate (clodronate). Calcif Tissue Int. 1997; 61: 59-61.
- Golub L, McNamara T, D’Angelo G, Greenwald R, Ramamurthy N. A nonantibacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J Dent Res. 1987; 66: 1310-1314.
- Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. 1997; 14: 216-248.
- Bodet C, Chandad F, Grenier D. Inhibition of host extracellular matrix destructive enzyme production and activity by a high-molecular-weight cranberry fraction. J Periodont Res. 2007; 42: 159-168.
- Ryan ME. Host Modulation: Conceptualization to Clinical Trials and Integration into Clinical Practice Journal of the California Dental Association. 2002; 30: 285-288.
- Bodet C, Piche M, Chandad F, Grenier D. Inhibition of periodontopathogenderived proteolytic enzymes by a high-molecular-weight fraction isolated from cranberry. Journal of Antimicrobial Chemotherapy. 2006; 57: 685-690.
- Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, et al. Minocycline reduces gingival collagenolytic activity during diabetes: preliminary observations and a proposed new mechanism of action. J Peridontal Res. 1983; 18: 516-526.
- Lovejoy B, Cleasby A, Hassell A, Longely K, Luther M, Weigl D, et al. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science. 1994; 263: 375-377.
- Smith G, Brandt K, Hasty K. Procollagenase is reduced to inactive fragments upon activation in the presence of doxycycline. Ann N Y Acad Sci. 1994; 732: 436-438.
- Golub L, Seigel K, Ramamurthy N, Mandel I. Some characteristics of collagenase activity in gingival crevicular fluid and its relationship to gingival disease in humans. J Dent Res. 1976; 55: 1049-1057.
- Kantarci A, Hasturk H, Van Dyke TE. Host-mediated resolution of inflammation in periodontal diseases. Periodontology. 2000; 40: 144-163.
- Golub LM, Suomalainen K, Sorsa T. Host modulation with tetracyclines and their chemically modified analogues. Curr Opin Dent. 1992; 2: 80-90.
