Abstract
Hemoglobin (Hb), a metalloprotein in Red Blood Cells (RBC), is highly conserved across all vertebrates and in some invertebrates. Each RBC houses approximately 250 million Hb molecules which serve as transporters of oxygen from the lungs to the tissues and carbon dioxide from the tissues to the lungs. However in the event of intravascular hemolysis, lysed RBCs release Hb into the circulation. A large fraction of this extracellular Hb forms complex with Haptoglobin (Hp) and is cleared from circulation by CD163, a macrophage surface receptor. Heme, a by-product of Hb oxidation, upon binding to Hemopexin (Hpx) is cleared by phagocytes in a similar manner. Once the scavenging capacity of Hp and Hpx reaches the saturation point, residual cell-free Hb and heme ensue into vascular dysfunctions and thrombogenic complications. Cell-free Hb also limits the bioavailability of Nitric Oxide (NO) and Carbon monoxide (CO), signalling molecules crucial for the maintainance of vascular architecture and hemostasis. Besides, studies have described that the oxidized form of Hb such as metHb or by-product(s) of heme are highly redox reactive and cause oxidative damage to surrounding tissues, resulting in initiation of pro-inflammatory and pro-coagulative cascades. On the other hand, cell-free Hb also manifests cytoprotective effect through modulation of Heme Oxygenase 1 (HO-1), ferritin and anti-oxidative response gene(s). In hemolytic diseases, accumulation of excess free Hb in plasma triggers pathophysiological events that are associated with adverse clinical outcomes such as acute and chronic vascular disease, in?ammation, thrombosis and renal impairment. Cell-free Hb also activates platelets via both direct and/or indirect mechanisms and promotes clinical events such as thrombosis and hypercoagulation. Apart from these complications, cell-free Hb also modulates phenotype and function of cells of both arms of the immune system- innate and adaptive. Monocytes, macrophages and neutrophils, which play important role in the first line of defense, are significantly affected when exposed to free Hb or heme. Similarly, the adaptive immune cells such as T and B lymphocytes also show altered response under hemolytic conditions. It has been demonstrated that patients with haemolytic disorders harbour altered immune cytome and response. This review briefly describes the current understanding of the effect of cell-free Hb on immune response in haemolytic disorders.
Keywords: Free haemoglobin; Heme; Monocytes; Macrophages; Neutrophils; B and T lymphocytes; Hemolytic diseases
Introduction
Intravascular hemolysis is characterized by the release of excess Hb into extracellular fluid. Extensive studies have reported the role of cell free Hb in the pathogenesis of many adverse clinical events including vascular dysfunctions, inflammations, thrombosis and renal impairments in hemolytic patients. Cell free Hb has been described as a potent facilitator of immune dysfunctions in patients with hemolyic disorders including Sickle Cell Disease (SCD), Paroxysmal Nocturnal Hemoglobinuria (PNH), Thalassemia, Hemolytic uremic Syndrome (HUS), Aplastic Anemia (AA) and Auto Immune Hemolytic Anaemia (AIHA).
In hemolytic disease conditions, the excessive accumulation of free Hb in blood or locally in tissues induces oxidative stress. The biological activity of free Hb can be either due to direct interaction of hemoglobin or heme with specific cellular receptors or it can have secondary effects due to heme breakdown mediated by Heme Oxygenase-1 (HO-1). The free Hb or its breakdown products such as iron and heme induce generation of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). The excessive ROS and RNS further modulate cellular processes such as protein oxidation, lipid peroxidation and nucleic acid oxidation, ultimately causing cellular dysfunctions [1]. The ROS and RNS also affect the immune system significantly [2,3]. The humoral compartment of innate immune system, immunoglobulins and complement system, also get affected by cell free Hb and heme [4-6]. Heme affects antigen binding properties of immunoglobulins [7]. These effects of Hb and heme on humoral component of immune system have recently been reviewed by Roumenina LT et al., 2016 [8]. The cellular component of innate immune cells such as monocytes, macrophages, Dendritic Cells (DCs) and neutrophils, which are associated with first line of defence mechanisms, have been described to have altered phenotypes and functions in response to hemolytic conditions. Besides, extensive studies have also described the effects of free Hb on the adaptive immune cells such as T and B lymphocytes and their altered responses in hemolytic diseases [9,10].
Monocytes and Macrophages in Hemolytic Disorders
Monocytes and macrophages are phagocytic cells and play a very crucial role in combating infections by engulfing microbes and also apoptotic cells. They also release inflammatory cytokines [11]. Monocytes originate from a common myeloid progenitor cell in the bone marrow and circulate in blood stream for 2 to 3 days before undergoing spontaneous apoptosis or migrating to various tissues where they are transformed into tissue macrophages, and reside there for months to years for defence responses [12,13]. The monocytes are also differentiated into DCs, the most potent professional Antigen- Presenting Cells (APCs) that orchestrate adaptive immune responses. While both monocytes and macrophages, originate from a common myeloid precursor and share functions in innate immunity, each has a very distinct life span finely tuned by the apoptotic caspases. Monocytes and macrophages play central roles in the initiation and resolution of inflammatory responses, principally through phagocytosis, release of cytokines, generation of ROS and via activation of acquired immune system [14].
In intravascular hemolysis, Hb is released into extracellular space and if not adequately sequestered, the pro-oxidant heme molecule (by-product of Hb) induces free radical formation, which promotes further tissue damage as well as inflammatory complications in hemolytic patients [15]. The Hb-mediated clinical consequences are well documented in diseases such as hemolytic anemia, SCD and PNH [16]. Under hemolytic conditions, free Hb forms complex with Haptoglobin (Hp) and Hb-Hp complex is cleared by scavenging receptor CD163 on monocytes and macrophages [17]. In other words, the CD163 serves a unique role of recycling the free Hb in hemolytic disease conditions [18,19]. Studies have reported the presence of CD163+ monocytes in circulation as well as locally at potential sites of intravascular hemolysis in hemolytic patients [20]. Another study has described a novel subset of CD68+ macrophages at the site of haemorrhage that bear high CD163 and low Human Leukocyte Antigen DR (HLA-DR) (CD68+CD163highHLA-DRlow). The cyto-protective properties of CD163high macrophages have been described showing their crucial involvement in scavenging free Hb [21]. Another report has shown that monocytes were differentiated towards an alternatively activated phenotype with high expression of CD163 and enhanced ability to scavenge free Hb in hemolytic conditions. These changes were found to be associated with stimulation of specific anti-oxidant and iron homeostasis pathways [22].
On the other hand, it is reported that free Hb synergistically increases production of pro-inflammatory cytokines including TNF-a by macrophages [23-25]. Further, a number of studies have also described the critical role of heme in enhancing release of TNF-a by macrophage during endotoxemia [26]. Reports also have shown the Hb-mediated enhancement in response of macrophages to TLR4-independent ligands of microbial origin, which was further suppressed by a plasma glycoprotein, Hemopexin (Hpx) [27]. Other study also has described that the Hpx therapy can revert the hemeinduced pro-inflammatory switching of macrophages in mice with SCD [28].
In a recent study, we have shown that a high number of proinflammatory (CD14+CD16hi) monocytes, which were positive for both intracellular Hb and platelets (CD42b) existed in patients with PNH and SCD. Our study also described that the CD14+ monocytes were transformed significantly into the CD14+CD16hi subsets after engulfing Hb-activated platelets in vitro. The CD14+CD16hi monocytes, positive for both intracellular Hb and CD42b, secreted significant TNF-a and IL-1β, unlike monocytes treated with only free Hb, which secreted more IL-10. Study therefore suggested that this alteration of monocyte phenotype may play a role in the increased propensity to pro-inflammatory/coagulant complications observed in these hemolytic disorders [29]. Other studies also have described the role of platelets in developing pro-inflammatory states through interactions with leukocytes in hemolytic patients [30]. Studies suggested that circulating platelets in SCD patients gets activated chronically, which may result in overall hypercoagulable states and pro-inflammatory events [31-33]. Other studies in SCD have described that the pro-inflammatory cytokines are mainly expressed in monocytes and macrophages upon stimulation from microbial infections.
Studies have described the delicate balance between the anti and pro inflammatory responses of these phagocytic cells. The hemegenerated ROS and other oxidative stress regulators were found to be playing the important roles in mediating pro-inflammatory responses of monocytes and macrophages in hemolytic patients [34]. On the other hand, scavenging of free Hb by these phagocytes is modulated by anti-inflammatory cytokines such as IL-4 and IL- 10, and enzyme HO-1 [35]. The above balance is altered when the intracellular oxidative stress increases and cells release elevated level of pro-inflammatory cytokines overcoming anti-oxidant barrier of mitochondria [34,36,37] and activating further the NF-kB pathways and releasing cytokines, which finally mediate leukocytesendothelium interactions and inflammations [37-39]. Extensive studies have suggested a close association between the altered innate responses and the development of chronic inflammatory conditions in hemolytic patients including SCD. Studies also have suggested the changes in phenotypes of monocytes and macrophages in patients with another hemolytic disorder such as PNH. The heme toxicity has been shown to be associated with the induction of pro-inflammatory phenotypes of the monocytes. Further, the glucocorticoid treatment skewed the monocyte differentiation into anti-inflammatory M2 phenotype with enhanced heme-iron recycling and anti-oxidant capacity [22].
Neutrophils in Hemolytic Disorders
Neutrophils as the most abundant granulocyte play very important role in the first line of immune defence. These phagocytic cells contain proteolytic enzymes such as elastase, myeloperoxidase, MMP9 and cathespsin G, and peptides and ROS precursors. Neutrophils are classically viewed as the mediators of inflammatory responses combating microbial infections [40]. Neutrophils are also known to mediate responses against intracellular molecules released in extracellular environment. These intracellular molecules harbour molecular patterns/signatures called Damage-Associated Molecular Patterns (DAMPs) [41]. In hemolytic disease conditions, neutrophils have been shown to react closely to DAMPs such as free Hb. Studies have shown the significant activation of neutrophils in hemolytic disease such as in SCD [42]. Several studies have described the neutrophil activation and impairement in response of these cells against infections in hemolytic disease conditions [43,44]. Upon exposure to free heme the impaired oxidative burst in neutrophils induced by HO-1 further increased the risk of infections in patients with SCD [45]. Furthermore studies have shown that the patients with PNH often exhibited neutrophil activation and neutropenia [46]. Research has suggested that the activation of neutrophils plays important role in immune responses but uncontrolled stimulation of neutrophils by the continuous presence of free Hb or heme in hemolytic conditions appears to be the significant contributor to the development of many chronic inflammatory consequences in hemolytic diseases. Extensive studies have described the crucial roles of free-Hb, ATP, mitochondrial DNA in activating neutrophils in these disease conditions [41]. The oxidised form of Hb, MetHb has been described binding to Toll-Like Receptor-2 (TLR2) and other ligands on neutrophils and promoting its activation [47]. On one hand, the free-Hb has been shown to inhibit apoptosis of neutrophils [48,49], on the other hand, free Hb promoted their activation via Protein Kinase C (PKC) pathway, which in turn induced migration of these cells [50]. Other studies also have described the activation of neutrophils during inflammation, which increased their life span and inhibited apoptosis [51].
Further, studies have described that as the scavenger of Nitric Oxide (NO) free Hb has increased the neutrophil activation and their binding to endothelium via PKC-mediated P-selectin recruitment on cell surface and also by elevating synthesis of Platelet Activating Factor (PAF) in endothelial cells [52]. Other studies have shown the heme-induced activation of neutrophils and the elevation in expression of VCAM-1, ICAM-1, P-selectin, E-selectin and vWF on endothelial cells, which finally mediated the attachment and extravasation of migrating neutrophils to tissues [53-56, 61]. The heme-mediated activation of neutrophils was regulated via TLR4 [56] and GPCR activation [63]. Besides, the activation of platelets and neutrophils by free Hb has been described elevating the plateletsneutrophils aggregates in circulation and instigating inflammations in hemolytic diseases such as in PNH [57,58] and SCD [57,58]. The constant exposure of neutrophils to free Hb alters the inflammatory responses resulting in uncontrolled secretion of cytokines and chemokines, which further instigates the inflammation of other leukocytes in hemolytic disorders. Therefore the better understanding of mechanisms of neutrophil activation by free Hb has potential to improve management and prognosis of hemolytic disorders.
T and B-Cell in Haemolytic Disorders
Antigen presenting cells such as DCs and macrophages present antigens to T-lymphocytes, which leads to antigen-specific activation of T cells. Multiplication of T-helper cells stimulates B-cells to produce antibodies against specific antigen. In this way, adaptive immune responses are initiated by the activated T cells. In hemolytic diseases, cell free Hb causes several cytotoxic effects on immune cells. Apart from activating platelets and monocytes free Hb also affects T-cell functions. Researchers have described that heme, a by-product of Hb, induced CD4+ T cell subset polarization to regulatory T cell (Treg) in purified naive-T cell/monocyte co-cultures from healthy donors through the monocyte anti-inflammatory heme-degrading enzyme HO-1 [64]. Previously this group has reported the reduction in Treg counts and B cell suppressive function along with elevated circulating IFN-γ, but lower IL-10 levels in allo-immunized SCD patients when compared with non-allo-immunized counterparts [9,10]. Other studies also have suggested altered phenotypes of T-cell in PNH patients. The peripheral T cells in PNH patients comprised a mixture of residual normal and Glycosyl Phosphatidyl Inositol (GPI)-deficient lymphocytes [65]. The lymphocytes of PNH patients lack the expression of many important GPI-anchored proteins that includes CD48, CD52, CD55, CD58, or CD59. The lymphocytes of PNH patients are usually identified in this manner [66]. In PNH patients, the two populations of normal and GPI-deficient T cells differ in their typical phenotypic characteristics. Richards et al. have drawn a comparison of these two populations and their study depicts significant level of reduction in the expression of HLA-DR on PNH T cells when compared to normal T cells existing in these patients. Their study also described the major differences in the distributions of naïve and memory T-cells between the two populations of normal versus PNH T-cell. The T-cells, which lack GPI-anchored proteins, are mainly naïve T cells, whereas, either normal or increased number of memory cells were found among normal T-cells of PNH patients. The expression of CD45RA antigen differed significantly between the normal and PNH T-cell clones. Thus, PNH T-cell clones show predominantly a naïve phenotype in comparison to normal T-cells. This group of researchers further extended their study to CD4+ (T-helper) and CD8+ (T-cytotoxic) lymphocyte subpopulations. In one third of PNH patients, the T-helper cells which are CD4+ exhibited a significant increase in the proportion of memory cell. This research group reported a very unique phenotype of PNH T cells. In consistent with having undergone thymic differentiation, the PNH T cells basically have a naïve (CD45RA+CD45R0-HLADR-) phenotype [67].
There are direct and indirect evidences which shows that the responses of T-cells against self-antigens can enforce damage of cells and tissues. TH1 cells by means of inappropriate activation of macrophages or direct responses of CD8+ cytotoxic T- lymphocytes can cause massive tissue damage. These T cell responses activate selfreactive B cells and this can initiate production of autoantibodies which are very harmful. CD4 regulatory T (Treg) cells exist in two forms natural and inducible. Both nTreg (natural) and iTreg (inducible) are known to play very important role in maintenance of immunologic tolerance and in the control of immune-mediated pathology [68, 69]. Approximately 5% of CD4+ T cells in humans comprised of natural (CD4+CD25+) Treg cells. During development in thymus the nTreg cells differentiate into suppressor cells which effectively inhibit autoreactive responses of effector T-cells [70,71]. Auto Immune Hemolytic Anaemia (AIHA) and Idiopathic Thrombocyto Penia (ITP) are hematologic diseases associated with the production of abnormal antibodies against RBCs and platelets respectively. AIHA is a case of autoimmunity in which self or autoantigen-specific Treg cells were recognized. Mostly AIHA patients have pathogenic autoantibody and activated autoreactive CD4+ Th1 cells which secrete IFN-γ and are specific for the Rh proteins on the membrane of RBCs [72]. In the peripheral blood and spleens of AIHA patients, Treg cells highly specific for epitopes on the Rh antigens were identified. These autoreactive T-cells secrete suppressive cytokines like IL-10 which inhibit the Th1 effector responses in vitro [73].
In T-cell dependent activation of the naïve B-cell, an APC presents a processed antigen to a T helper cell and also B cell presents the same antigen to the primed Th cell. In response, cytokines are released from T cell which further activates B cells. B cells are also activated in T cell independent manner, in which mature B cells reacts to highly repetitive structures. This instigates B cell receptors cross-linking on the surface of B cells. Plasma cells that are derived from the B cells synthesize and secrete antibodies [74]. In hemolytic diseases particular in AIHA, antibodies secreted from B cells are directed against the individual’s own cells causing immature lysis of RBCs and accumulation of free Hb [75,76]. IgG autoantibody produced in AIHA that accounts for warm AIHA development, specifically needs participation of T helper cells for B cell responses. Among the various cytokines from IL-1 super- family, IL-33 is a newly described cytokine and is known to be involved in T cell mediated immune responses [77]. A group of researchers recently reported that serum IL-33 levels was positively associated and hence contributing to increased anti-RBC autoantibody levels in AIHA patients [78]. Hence, in clinical practice, targeting cytokines like IL- 33 suggest a valuable therapeutic strategy for AIHA patients where presence of harmful autoantibodies contributes to disease severity and pathogenesis.
Conclusion
In hemolytic diseases, the lysis of RBCs releases free Hb whose metabolism causes accumulation of heme and ROS (generated due to Hb degradation) in extracellular spaces or in plasma. The above molecules alter many physiological functions including that of immune system. Free Hb and heme affect humoral compartment of immune system and also activate the immnue cells including monocytes, macrophages and DCs, which are associated with the first line of immune responses. The monocytes and macrophages exhibit both anti-inflammatory and pro-inflammatory response in hemolytic conditions. Studies have described that the monocytes developed antiinflammatory phenotypes after engulfing free Hb, while they were transformed into pro-inflammatory lineages when they engulfed Hbactivated platelets. Further, studies have described the activation of neutrophils by free Hb or heme in hemolytic disorders. Furthermore the excessive heme uptake by neutrophils induced the oxidative burst mediated by HO-1 during granulopoiesis observed in SCD. Studies have shown the heme mediated-polarization of CD4+ T cell subset to regulatory T cell in hemolytic disorders. The immunophenotypic characteristics of GPI-deficient populations of T cells in PNH patients showed significantly lower levels of HLA-DR expression as compared to coexisting normal T cells. The PNH T cells comprised of mainly naïve T cells; on the other hand healthy donor T cells showed either normal or increased proportions of memory cells. The review thus describes the effect of free Hb and its by-products on the phenotypes and functions of immune cells (mentioned in schematic Figure 1), highlighting the need of further understanding of the complex pathophysiology of the immune system under hemolytic conditions to improvie therapeutic management of hemolytic disorders.
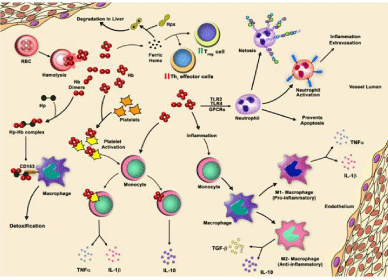
Figure 1: Schematic representation of immune response to free Hb and heme in hemolytic conditions. Intravascular hemolysis releases Hb into the plasma,
which forms complex with Haptoglobin (Hp) and is subsequently cleared from circulation following binding to CD163 receptor on monocytes and macrophages.
Similarly, heme (by-product of Hb degradation) is removed from circulation by binding to scavenger protein Hemopexin (Hpx). The free heme increases number of
regulatory T cells (Treg) and decreases helper T cells (Th1). The free Hb interacts with neutrophil and promotes its activation, which further leads to inflammation
and extravasation. In response to free Hb, neutrophils form NET (Neutrophil Extracellular Traps) to trap infectious agents. The free Hb increases the life span of
neutrophils and prevents apoptosis. Further, the monocytes are transformed into pro-inflammatory subsets or differentiated into M1 macrophages, when engulf Hbactivated
platelets. On the other hand, monocytes are transformed into anti-inflammatory subsets or differentiated into M2 macrophages, when engulf only free Hb.
Acknowledgements
Authors thank Ms. Sulagna Bhattacharya of Reginal Centre for Biotechnology for editing the manuscript.
References
- Nathan C, Ding A. SnapShot: reactive oxygen intermediates (ROI). Cell. 2010; 140: 951-951.
- Wink DA, Hines HB, Cheng RY, Switzer CH, Santana WF, Vitek MP et al. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011; 89: 873-891.
- Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009; 66: 121-127.
- Green D, Furby FH, Berndt MC. The interaction of the factor VIII/von Willebrand factor complex with hematin. Thromb. Haemost. 1986; 56, 277-282.
- Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998; 92: 3983-3996
- Repesse Y, Dimitrov JD, Peyron I, Farrokhi Mooshai E, Kiger L, Dasgupta S, et al. Heme binds to factor VIII and inhibits its interaction with activated factor IX. J. Thromb. Haemost. 2012; 10: 1062-1071
- McIntyre JA. The appearance and disappearance of antiphospholipid autoantibodies subsequent to oxidation–reduction reactions. Thromb. Res. 2004; 114: 579-587
- Roumenina LT, Rayes J, Lacroix-Desmazes S, Dimitrov JD. Heme: Modulator of Plasma Systems in Hemolytic Diseases. Trends Mol Med. 2016; 22: 200-213.
- Bao W, Zhong H, Li X, Lee MT, Schwartz J, Sheth S, et al. Immune regulation in chronically transfused allo-antibody responder and nonresponder patients with sickle cell disease and thalassemia major. Am. J. Hematol. 2011; 86: 1001-1006
- Bao W, Zhong H, Manwani D, Vasovic L, Uehlinger J, Lee MT, et al. Regulatory B-cell compartment in transfused alloimmunized and non-alloimmunized patients with sickle cell disease. Am. J. Hematol. 2013; 88: 736-740.
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity and relationship with dendritic cells. Annu. Rev. Immunol. 2009; 27: 669-692.
- Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006; 27: 244-250.
- Fahy RJ, Doseff AI, Wewers MD. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J. Immunol. 1999; 163: 1755-1762.
- Parihar A, Eubank TD, Doseff AI. Monocytes and Macrophages Regulate Immunity through Dynamic Networks of Survival and Cell Death. J Innate Immun. 2010; 2: 204-215.
- Landis C. Why the inflammatory response is important to the cardiac surgical patient. J Extra Corpor Technol. 2007; 39: 281-284.
- Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA. 2005; 293: 1653-1662.
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, et al. Identification of haemoglobin scavenger receptor. Nature 2001; 409: 198-201.
- Davis BH, Zarev PV. Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry B Clin Cytom. 2005; 63: 16-22.
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: An immunologic functional perspective. Annu Rev Immunol. 2009; 27: 451-483.
- Schaer CA, Vallelian F, Imhof A, Schoedon G, Schaer DJ. CD163-expressing monocytes constitute an endotoxin-sensitive Hb clearance compartment within the vascular system. J. Leukoc. Biol. 2007; 82: 106-110.
- Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009; 174: 1097-1108.
- Vallelian F, Schaer CA, Kaempfer T, Gehrig P, Duerst E, Schoedon G, et al. Glucocorticoid treatment skews human monocyte differentiation into a hemoglobin-clearance phenotype with enhanced heme-iron recycling and antioxidant capacity. Blood. 2010; 116: 5347-5356.
- Roth RI, Kaca W, Levin J. Hemoglobin: a newly recognized binding protein for bacterial endotoxins (LPS) Prog Clin Biol Res. 1994; 388: 161-172.
- Su D, Roth RI, Yoshida M, Levin J. Hemoglobin increases mortality from bacterial endotoxin. Infect Immun. 1997; 65: 1258-1266.
- Kaca W, Roth RI, Levin J. Hemoglobin, a newly recognized lipopolysaccharide (LPS)-binding protein that enhances LPS biological activity. J Biol Chem. 1994; 269: 25078-25084.
- Yang H, Wang H, Bernik TR, Ivanova S, Wang H, Ulloa L et al. Globin attenuates the innate immune response to endotoxin. Shock. 2002; 17: 485-490.
- Lin T, Kwak YH, Sammy F, He P, Thundivalappil S, Sun G, et al. Synergistic inflammation is induced by blood degradation products with microbial Toll-like receptor agonists and is blocked by hemopexin. J Infect Dis. 2010; 202: 624-632.
- Vinchi F, Costa da Silva M, Ingoglia G, Petrillo S, Brinkman N, Zuercher A, et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016; 127: 473-486.
- Singhal R, Chawla S, Rathore DK, Bhasym A, Annarapu GK, Sharma V, et al. Development of pro-inflammatory phenotype in monocytes after engulfing Hb-activated platelets in hemolytic disorders. Clin Immunol. In communication.
- Maugeri N, Baldini M, Ramirez GA, Rovere-Querini P, Manfredi AA. Platelet-leukocyte deregulated interactions foster sterile inflammation and tissue damage in immune-mediated vessel diseases. Thromb Res. 2012; 129: 267-273.
- Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007; 110: 2166-2172.
- Stokes KY, Granger DN. Platelets: A critical link between inflammation and microvascular dysfunction. J Physiol. 2012; 590: 1023-1034.
- Chiang EY, Frenette PS. Sickle cell vaso-occlusion. Hematol/Oncol Clin North Am, 2005; 19: 771-784.
- Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mito-chondrial reactive oxygen intermediates and their in-volvement in cytotoxicity. Proc Natl Acad Sci USA 1995; 92: 8115-8119.
- Muhl D, Woth G, Drenkovics L, Varga A, Ghosh S, Csontos C, et al. Comparison of oxidative stress & leukocyte activation in patients with severe sepsis & burn injury. Indian J Med Res. 2011; 134: 69-78.
- Read MA, Whitley MZ, Williams AJ, Collins T. Nf-kappa B and I kappa B alpha: An inducible regulatory system in endothelial activation. J Exp Med. 1994; 179: 503-512.
- Patel SJ, Jindal R, King KR, Tilles AW, Yarmush ML. The inflammatory response to double stranded DNA in endothelial cells is mediated by Nf kappab and Tnf alpha. PloS One. 2011.
- Croizat H. Circulating cytokines in sickle cell patients during steady state. British J Haematol. 1994; 87: 592-597.
- Raghupathy R, Haider MZ, Azizieh F, Abdelsalam R, D'Souza TM, Adekile AD. Th1 and Th2 cytokine profiles in sickle cell disease. Acta Haematol. 2000; 103: 197-202.
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012; 30: 459-489.
- Pittman K, Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013; 5: 315-323.
- Lard LR, Mul FP, de Haas M, Roos D, Duits AJ. Neutrophil activation in sickle cell disease. J Leukoc Biol. 1999; 66: 411-415.
- Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J Infect Dis. 2010; 14.
- Cunnington AJ, de Souza JB, Walther M, Riley EM. Malaria impairs resistance to Salmonella through heme- and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat Med. 2011; 18: 120-127
- Evans C, Orf K, Horvath E, Levin M, De La Fuente J, Chakravorty S, et al. Impairment of neutrophil oxidative burst in children with sickle cell disease is associated with heme oxygenase-1. Haematologica. 2015; 100: 1508-1516.
- Socié G, Mary JY, de Gramont A, Rio B, Leporrier M, Rose C, et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet. 1996; 348: 573-577.
- Lee SK, Goh SY, Wong YQ, Ding JL. Response of Neutrophils to Extracellular Haemoglobin and LTA in Human Blood System. EBioMedicine. 2015; 2: 225-233
- Arruda MA, Rossi AG, de Freitas MS, Barja-Fidalgo C, Graša-Souza AV. Heme inhibits human neutrophil apoptosis: involvement of phosphoinositide 3-kinase, MAPK, and NF-kappaB. J Immunol. 2004; 173: 2023-2030.
- Arruda MA, Barcellos-de-Souza P, Sampaio AL, Rossi AG, Graça-Souza AV, Barja-Fidalgo C. NADPH oxidase-derived ROS: key modulators of heme-induced mitochondrial stability in human neutrophils. Exp Cell Res. 2006; 312: 3939-3948
- Graça-Souza AV, Arruda MA, de Freitas MS, Barja-Fidalgo C, Oliveira PL. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002; 99: 4160-4165.
- Witko-Sarsat V, Pederzoli-Ribeil M, Hirsch E, Sozzani S, Cassatella MA. Regulating neutrophil apoptosis: new players enter the game. Trends Immunol. 2011; 32: 117-124.
- . Okayama N, Coe L, Itoh M, Alexander JS. Exogenous nitric oxide increases neutrophil adhesion to cultured human endothelial monolayers through a protein kinase G dependent mechanism. Inflammation. 1999; 23: 37-50.
- Wagener FA, Feldman E, de Witte T, Abraham NG. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997; 216: 456-463
- Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014; 123: 377-390
- von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012; 209: 819-835.
- Frimat M , Tabarin F, Dimitrov JD, Poitou C, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, et al. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood. 2013; 122: 282-292.
- Annarapu GK, Singhal R, Peng Y, Guchhait P. Inhibition of Hb Binding to GP1ba Abrogates Hb-Mediated Thrombus Formation on Immobilized VWF and Collagen under Physiological Shear Stress. PLoS One. 2016; 11: e0154276.
- Singhal R, Annarapu GK, Pandey A, Chawla S, Ojha A, Gupta A, et al. Hemoglobin interaction with GP1ba induces platelet activation and apoptosis: a novel mechanism associated with intravascular hemolysis. Haematologica. 2015; 100: 1526-1533.
- Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007; 110: 2166-2172.
- Polanowska-Grabowska R, Wallace K, Field JJ, Chen L, Marshall MA, Figler R, et al. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler Thromb Vasc Biol. 2010; 30: 2392-2399
- Monteiro AP, Pinheiro CS, Luna-Gomes T, Alves LR, Maya-Monteiro CM, Porto BN, et al. Leukotriene B4 mediates neutrophil migration induced by heme. J Immunol. 2011; 186: 6562-6567
- Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007; 282: 20221-20229
- Porto BN, Alves LS, Fernández PL, Dutra TP, Figueiredo RT, Graça-Souza AV, et al. Heme induces neutrophil migration and reactive oxygen species generation through signaling pathways characteristic of chemotactic receptors. J Biol Chem. 2007; 282: 24430-24436.
- Zhong H, Bao W, Friedman D, Yazdanbakhsh K. Hemin controls T cell polarization in sickle cell alloimmunization. The Journal of Immunology. 2014; 193: 102-110.
- Richards SJ, Norfolk DR, Swirsky DM, Hillmen P. Lymphocyte subset analysis and glycosylphosphatidylinositol phenotype in patient with paroxysmal nocturnal hemoglobinuria. Blood. 1998; 92: 1799-1806.
- Tseng JE, Hall SE, Howard TA, Ware RE. Phenotypic and functional analysis of lymphocytes in paroxysmal nocturnal hemoglobinuria. Am J Hematol. 1995; 50: 244-253.
- Stephen J. Richards, Gareth J. Morgan, and Peter Hillmen. Analysis of T Cells in Paroxysmal Nocturnal Hemoglobinuria provides direct evidence that Thymic T-Cell production declines with age. Blood. 1999; 94: 2790-2799
- Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003; 171: 6323-6327.
- Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006; 212: 203-216.
- Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFNalpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001; 166: 5530-5539.
- McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002; 195: 221-231.
- Barker RN, Hall AM, Standen GR, Jones J, Elson CJ. Identification of T-cell epitopes on the Rhesus polypeptides in autoimmune hemolytic anemia. Blood. 1997; 90: 2701-2715.
- Hall AM, Ward FJ, Vickers MA, Stott LM, Urbaniak SJ, Barker RN. Interleukin-10-mediated regulatory T-cell responses to epitopes on a hu- man red blood cell autoantigen. Blood. 2002; 100: 4529-4536.
- Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: the immune system in health and disease. Current Biology. 1997.
- Shoenfeld Y, Cervera R, Gershwin ME. Diagnostic criteria in autoimmune diseases. Springer Science & Business Media. 2010.
- Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. American journal of hematology. 2002; 69: 258-271.
- Wang S, Ding L, Liu SS, Wang C, Leng RX, Chen GM, et al. IL-33: a potential therapeutic target in autoimmune diseases. J Investig Med. 2012; 60: 1151–1156.
- Bu X, Zhang T, Wang C, Ren T, Wen Z. IL-33 reflects dynamics of disease activity in patients with autoimmune hemolytic anemia by regulating autoantibody production. Journal of translational medicine. 2015; 13.
