Abstract
Introduction: Postpartum Hemorrhage (PPH)-induced coagulopathy should be better explored. An innovative Simultaneous Generation of Thrombin and Plasmin Assay (SGTPA) was developed.
Objective: To establish postpartum SGTPA references.
Methods: Blood samples collected immediately after delivery (T0) and then 30, 60, 120 and 360 minutes later. Thrombin Generation (TG) and Plasmin Generation (PG) changes over time analyzed in 51 women after elective cesarean section without PPH and compared with Non-Pregnant (NP) women. The SGTPA variables’ correlations with fibrinogen levels, D-dimer levels and physiological blood loss were assessed in a mixed model.
Results: 37 women were included. TG and PG were higher in the postpartum group than in the NP group. TG increased early and then remained stable (baseline TG Area Under the Curve (AUC) [95% Confidence Interval (CI)] = 41037 [36850-43537] nM.min). The fibrinogen level varied over time, along with TG (p<0.001). Plasmin generation increased from 30 to 120 minutes (AUC [95% CI]: 2104 [1437; 2613] nM.min), along with a change in the D-dimer level (p=0.018). The time to the plasmin peak and the time interval between the TG and PG peaks showed the greatest inter-individual variability at T0 and were associated with physiological volumes of blood loss during cesarean delivery.
Conclusion: Reference SGTPA postpartum range was established. The SGTPA appears to be valuable for measuring TG and PG. TG and coagulation activation increased immediately after delivery, whereas PG and fibrinolysis increased later.
Keywords: Postpartum; Fibrinolysis; Plasmin; D-dimers; Fibrinogen; Thrombin
Key Points
• The simultaneous generation of thrombin and plasmin assay appeared to be reliable in a population of women without postpartum hemorrhage. Levels of thrombin and plasmin generation were higher than in non-pregnant women.
• Thrombin generation started immediately after delivery and then remained stable, whereas plasmin generation started 30 to 120 minutes after delivery. The time to the plasmin peak and the time interval between the thrombin and plasmin peaks were associated with the occurrence of physiological postpartum bleeding.
Introduction
Fibrinolysis is a complex physiological system involved in massive bleeding disorders [1,2]. Tranexamic Acid (TA) (Exacyl®, Sanofi, France) is an antifibrinolytic drug that inhibits Plasmin Generation (PG) by competitively blocking the native fibrin lysine binding sites on the kringle 5 domain of activated plasminogen [1,2]. TA reduces bleeding and the transfusion requirement in major surgery and trauma [3,4]. Hyperfibrinolysis was previously identified as a part of Postpartum Hemorrhage (PPH)-induced coaguloapthy in an open labelled controlled study demonstrating a significant early increase in levels of D-dimer and Plasmin-Antiplasmin (PAP) complex, maximal at the second hour after delivery [5]. A high dose of TA inhibited this hyperfibrinolysis and reduced PPH volume and duration and maternal morbidity since the 30th minute until the second hour after administration [5,6]. An international doubleblind, placebo-controlled, randomized clinical trial (WOMAN) demonstrated a reduction of maternal mortality due to bleeding after a single 1g dose of TA dose [7]. In a one center-study of the WOMAN trial including 167 women experiencing PPH, hyperfibrinolysis was detected as ex-vivo thromboelastometric maximal lysis >15% in 35 (23%) [8]. The tranexamic acid impact was demonstrated on the D-dimers’s level decrease: The mean (SD) D-dimer concentration was 7.1 (7.0) mg/l in TA-group and 9.6 (8.6) mg/l in placebo-group (p=0.09). After adjusting for baseline, the D-dimer concentration was 2.16mg/l lower in TXA-treated women (-2.16, 95% CI -4.31 to 0.00, (p=0.05)). There was no significant difference in ML between TXA- and placebo-treated women (12.3% (18.4) and 10.7% (12.6), respectively; p=0.52) nor on any other parameters [9]. Although TA is clearly effective, the optimal dose for administration during Cesarean Section (CS) with a risk of PPH has yet to be determined. Tranexamic Acid to Reduce Blood Loss in Hemorrhagic Caesarean Delivery (TRACES) dose-ranging placebo-controlled trial was designed to reach this objective [10,11]. An innovative assay has been developed to improve hyperfibrinolysis detection and appreciate its intensity, adapted from the Van Geffen et al. method [11]. This novel hemostasis assay (NHA, referred to here as the Simultaneous Generation of Thrombin and Plasmin Assay (SGTPA)) measured Thrombin Generation (TG), Plasmin Generation (PG) and the interaction between the two in the general population and in patients with rare bleeding disorders [11,12]. Thrombin generation has been previously measured in normal pregnancy and preeclamptic patients [13,14], in order to predict thrombotic risk [15] or to detect PPHassociated coagulopathy [16]. Plasmin generation cannot be assayed directly, given plasmin’s short half-life in plasma. In the TRACES trial, SGTPA is being used to provide additional data on acute obstetric coagulopathy and on TA’s dose-dependent antifibrinolytic impact during hemorrhagic vs. non-hemorrhagic Cesarean Section (CS) [9,10]. The objective of the present sub-study of the TRACES dataset was to use the SGTPA to establish reference ranges for TG, PG and other biomarkers of coagulation and fibrinolysis in the group of women without PPH [9,10].
Materials and Methods
The TRACES multicenter DBPC RCT was approved by an investigational review board (CPP Nord Ouest IV, Lille France; reference: 15/50_020216) [9].
Study design
An ancillary study of the TRACES dataset explored the pharmacokinetics and pharmacodynamics of TA during PPH after CS and was funded by the French National Agency for Medicines and Healthcare Products (Agence nationale de sécurité du médicament et des produits de santé, ANSM) [10]. In the TRACES study, the SGTPA and conventional laboratory tests are used to investigate TA’s antifibrinolytic effect vs. placebo during PPH after CS [11]. Hence, the present “TRACES non-PPH” unblinded ancillary study was designed to provide SGTPA reference values after an elective, nonhemorrhagic CS (Appendix 1) [9]. Four centers participated in this ancillary study, which was promoted by Lille University Medical Center (Lille, France).
Population
We included patients undergoing elective CS with normal bleeding (blood loss <500mL) (Appendix 1). All patients received comprehensive information on the study’s objectives and procedures and gave their written consent to participation. The main exclusion criteria were age under 18, a lack of social security coverage, difficult venous access, and unexpected perioperative bleeding.
We recorded clinical data, obstetric data and the volume of blood loss, together with laboratory test results for blood samples collected upon delivery (T0) and then 30 (T30), 60 (T60), 120 (T120) and 360 (T360) minutes and 2 (±1) and 42 (±14) days thereafter (Appendix 1).
The present pilot study included 51 patients undergoing an elective, non-hemorrhagic CS after a normal pregnancy in 4 centers from March 2016 to June 2019. Thirteen of the 51 patients were then excluded due to PPH (blood loss >500mL: n=3), a disease condition affecting the mother or the fetus (uterine fibroids, and small for gestational age: n=3), or failure to freeze the patients’ samples (n=7, in one center). A total of 37 patients (Non-PPH group) met the inclusion criteria, provided all samples and attended follow-up visits (Figure 2). The study population’s anthropometric and obstetric characteristics are summarized in Table 1.
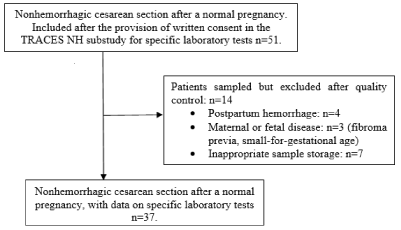
Figure 2: Flow Chart.
Non-hemorrhagic cesarean section
n=37
Maternal data
Age (years)
33.6 (4.5)
Weight (kg)
Before pregnancy
61.0 (56.0 to 72.0)
At the end of pregnancy
75.0 (66.0 to 84.0)
Height (cm)
164 (6.2)
Parity (n primiparous (%))
13 (35.1)
Peroperative bleeding (mL)
250 (150 to 382)
Additional postpartum bleeding (T0-T360) (mL)
170 (110 to 230)
Neonatal data
Weight (g)
3410 (3150 to 3690)
Apgar score at 1/5/10 minutes
10/10/10 (10 to 10)
Arterial blood pH
7.2 (0.1)
Venous blood pH
7.3 (0.1)
Data are expressed as the mean (SD) or median [IQR].
Table 1: Anthropometric and obstetrical data.
Biology
The sample processing steps were performed with caution. Blood samples were immediately sent to each center’s central laboratory. The plasma was separated by centrifugation (at 2500g for 15 minutes, 20°C) and frozen at -70°C. Frozen aliquots were then transferred to Lille University Medical Center’s central laboratory by an approved courier. Non-specific laboratory tests (a complete blood count, a platelet count, coagulation screening [including the activated partial prothrombin time, the prothrombin time, fibrinogen, factors II and V, antithrombin, fibrin monomers and D-dimers] and kidney function variables) were carried out in each center, using standard procedures, devices and reference value.
The SGTPA (adapted from Van Geffen et al.’s NHA) and assays for Thrombin-Antithrombin (TAT) complexes (Enzygnost® TAT micro, Siemens Healthineers, Marburg, Germany) and PAP complexes (Technozym® PAP complex ELISA kit, Technoclone, Vienna, Austria) were performed at the central laboratory in Lille. TG and PG were measured in a fluorimeter (Fluoroskan Ascent®, Thermo-Labsystems, Helsinki, Finland) using black polystyrene 96- well microtiter plates (Thermo-Labsystems). We used 50mM Trisbuffered saline (TBS) prepared from saline (Euromedex), Trizma Base® and Trizma hydrochloride® (Sigma, Germany). The TBS was then filtered through a 0.8μm Millex filter; calcium chloride 200mM (VWR Prolabo®), filtered as well. We also used recombinant activated tissue factor (TF, Innovin®, Siemens Healthineers); cephalin (CK®-Prest®, Diagnostica Stago, Asnières-sur-seine, France); tissue plasminogen activator (t-PA, Alteplase®, Boehringer Ingelheim, Ingelheim am Rhein, Germany), pure human thrombin and human plasmin (Enzyme Research Laboratories, distributed by Kordia, Leiden, the Netherlands) as calibrators, the thrombin-specific substrate Bz- β-Ala-Gly-Arg-7-amino-4-methylcoumarin, and plasmin specific substrate bis-(CBZ-L-phenylalanyl-L-arginine amide)-rhodamine (both from Chiralix, Nijmegen, the Netherlands).
Additional technical details are provided in Appendix 2 and Figure S1.
To describe the proteolytic activity of both thrombin and plasmin, several variables were defined and automatically calculated using an Excel® macro. The variables were shown in Figure 1 for a normal pooled-plasma sample.
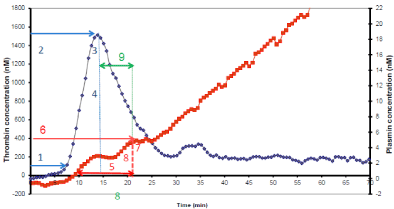
Figure 1: Simultaneous thrombin and plasmin generation assay parameters.
Simultaneous thrombin (TG) and plasmin (PG) generation in a single well.
From a normal pooled plasma. TG signal was divided in four parameters:
(1) TG lagtime (min); (2) TG peak time (min); (3) TG peak height (nM); (4)
TG area under the curve (AUC) (nM/min). PG signal was divided in four
parameters: (5) Fibrin Lysis Time (FLT) (min); (6) PG peak time (min); (6) PG
peak height (nM); (8) PG area under the curve (AUC) (nM/minute). Interaction
between thrombin and plasmin was expressed as (9) Delta between the
thrombin and plasmin peak times (min).
For TG, four variables were determined:
• The TG lag time (in min), i.e. the time between reaction initiation and the start of TG.
• The thrombin peak time (in min), i.e. the time at which the TG peak occurred.
• The thrombin peak (nM), corresponding to the maximal velocity of TG.
• The area under the curve (AUC, in nM.min) for TG, i.e. the total thrombin generated.
For PG, four other variables were defined:
• The fibrin lysis time (FLT) (in min), i.e. the interval time between the start of PG and its peak.
• The plasmin peak (in nM) i.e. the PG when the curve shifted from a convex shape to a linear shape, representing lysis of the clot by plasmin.
• The plasmin peak time (in min), i.e. the time when PG reached its greatest velocity.
• The area under the curve (AUC, in nM.min) for PG, between fibrinolysis onset and the plasmin peak.
The interaction between TG and PG was probed with the following variable:
• The time interval between the plasmin peak and thrombin peak.
Laboratory standards for the SGTPA had been established using samples from 30 healthy volunteers. For comparison with postpartum values, we focused here on the data from 7 Non-Pregnant (NP) women of childbearing age. Intra- and inter-assay dispersions were measured as a means of quality control.
Statistical methods
Computerized data were collected and managed by the Epidemiology and Public Health Biostatistics Unit at Lille University Medical Center. The study data were analyzed in accordance with the MR 06001 reference methodology, as defined by the French National Data Protection Commission (Commission nationale de l’informatique et des libertés (Paris, France)).
The sample size calculation for the TRACES_non-PPH study was based on the number of data points needed to validate the SGTPA (n=45) [11] and the number needed to evaluate fibrinolysis inhibition in the TRACES trial (n=48) [5,6,9,10]. Statistical analyses were performed at the University of Lille’s Biostatistics Department (under the responsibility of Professor Alain Duhamel, using SAS software (SAS Institute Inc, Cary, NC). All statistical tests were two-tailed, and the threshold for statistical significance was set to p<0.05.
Continuous variables were expressed as the Mean ± Standard Deviation (SD) or (for non-normally distributed data) the median (interquartile range). Normality of distribution was assessed using histograms and the Shapiro-Wilk test. The SGTPA’s reference values in the non-PPH vs. non-pregnant groups compared using a Mann- Whitney test. To study the change over time in the SGTPA variables and other test results from baseline and between each time point (at T0, T30, T60, T120, and T360), we used a linear mixed models with a random intercept (to account for the correlation between samples obtained for the same patient). Bonferroni method’s was used to adjust for multiple comparisons. Non-normally distributed variables were rank-transformed before assessment in the same models. We also used a linear mixed model with a random intercept to analyze the impact of changes over time in laboratory test results and SGTPA variables; the laboratory test results and time were included as fixed effects. Associations between the changes over time in pairs of variables were quantified as an estimated Effect Size (ES). Again, non-normally distributed variables were rank-transformed before assessment in the same models. Lastly, we evaluated the correlation between SGTPA variables with ideal weight [24], total blood loss, and additional blood loss at baseline by calculating Pearson’s correlation coefficient or (for non-normally distributed variables) Spearman’s rank correlation coefficient.
Results
Overall, the SGTPA values at T0 were higher in the non-PPH group than in the non-pregnant group. The thrombin peak, the thrombin AUC and the plasmin peak were significantly higher (p=0.002, p<0.001 and p=0.010, respectively) (Table 2). The TG and PG postpartum reference values at the various time points postpartum are shown in Figure 3 and Appendix 3.
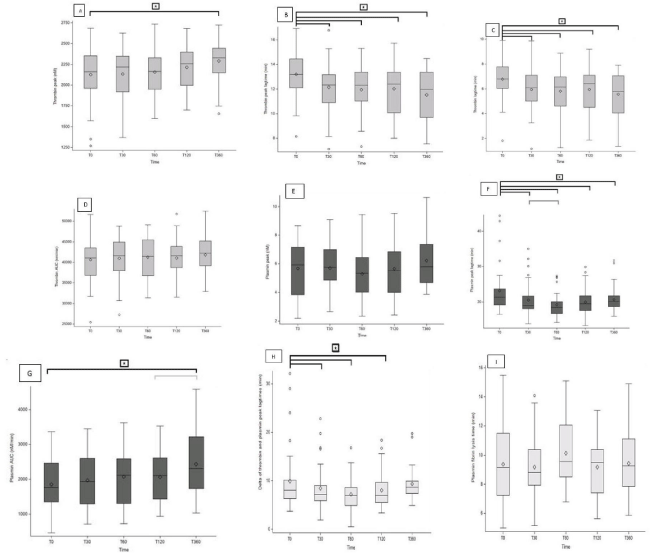
Figure 3: Distribution of Simultaneous Generation of Thrombin and Plasmin parameters at each time. 3A: Thrombin peak; 3B: Thrombin peak lagtime; 3C:
Thrombin lagtime; 3D: Thrombin AUC; 3E: Plasmin peak; 3F: Plasmin peak lagtime; 3G: Plasmin AUC; 3H: Delta of the thrombin and plasmin peaks lagtimes; 3I:
Fibrin lysis time.
The distribution of the SGTPA variables at each time point. 3A: The thrombin lag time; 3B: The thrombin peak time; 3C: The thrombin peak; 3D: The thrombin AUC;
3E: The fibrin lysis time; 3F: The plasmin peak time; 3G: The plasmin peak; 3H: the plasmin AUC; 3I: the difference between the thrombin and plasmin peak times.
The SGTPA was performed in a single well, using normal pooled plasma. The TG signal was tracked according to four variables: the TG lag time (min), the TG
peak time (min), the TG peak height (nM), and the TG area under the curve (AUC) (nM.min). The PG signal was tracked according to four variables: the fibrin lysis
time (FLT) (min), the PG peak time (min), the PG peak height (nM), and the PG AUC (nM.min). The interaction between thrombin and plasmin was expressed as
the difference between the thrombin and plasmin peak times (min).
The boxes in the Tukey box plot show the 25th, 50th and 75th percentiles, the whiskers indicate values outside the lower and upper quartile (length: 1.5 x interquartile
range), and the diamond indicates the mean. A black bracket indicates significant differences from T0. A grey bracket indicates significant differences between each
time point. Values were compared in a post-hoc pairwise linear mixed model (corrected for multiple comparisons, using Bonferroni’s method).
Variable
group
non-PPH/NP comparison
SGTPA variable (unit)
non-PPH
N=36NP
N=7p value
Thrombin lag time (min)
Median [IQR]
6.8 [6.0; 7.8]
6.4 [4.7; 7.5]
0.64
Range
1.8 | 9.9
4.6 | 11.3
Thrombin peak lag time (min)
Median [IQR]
13.2 [12.1; 14.5]
12.2 [10.1; 13.4]
0.32
Range
8.2 | 16.9
9.9 | 18.6
Thrombin peak (nM)
Median [IQR]
2160 [1963; 2354]
1415 [1308; 16]1]
0.002
Range
1268 | 2686
1128 | 2070
Thrombin AUC (nM.min)
Median [IQR]
41037 [36850; 43537]
27493 [25814; 32023]
<0.001
Range
25447 | 51631
25300 | 36858
Fibrin lysis time (min)
Median [IQR]
9.1 [7.2; 11.5]
8.2 [6.0; 9.1]
0.13
Range
5.0 | 15.5
5.0 | 10.1
Plasmin peak time (min)
Median [IQR]
21.4 [19.1; 23.8]
16.3 [16.0; 18.7]
<0.001
Range
16.6 | 44.3
16.0 | 19.2
Plasmin peak (nM)
Median [IQR]
5.9 [3.9; 7.2]
3.4 [2.6; 4.2]
0.01
Range
2.2 | 8.7
2.4 | 4.5
Plasmin AUC (nM.min)
Median [IQR]
1766 [1351; 2464]
1620 [1592; 3027]
0.64
Range
463.6 | 3371
1323 | 3547
Difference between thrombin and plasmin peaks (min)
Median [IQR]
8.1 [6.3; 10.2]
5.1 [2.6; 6.3]
0.002
Range
3.7 | 32.1
0.5 | 6.8
Values are expressed as the median [IQR] (range). Groups were compared using a Wilcoxon non-parametric test. Abbreviations: SGTPA: Simultaneous Generation of Thrombin Plasmin Assay; non-PPH: Non-hemorrhagic, NP=Non-Pregnant.
Table 2: SGTPA variables recorded at inclusion in the non-PPH postpartum group and reference values in Non-Pregnant (NP) women.
When validated against a normalized plasma pool, the intra-assay and inter-assay coefficients of variation were low for each SGTPA variable, with the quality care level. The intra-individual (betweentimes) and the inter-individual (between-observations) dispersions for TG in the non-PPH group different slightly from those measured in the control group: the coefficient of variation for the baseline TG AUC was 13 %. The PG variables varied more than the TG variables at all time points. The greatest coefficient of variation was observed for the plasmin peak time at T0 (Figure 3, Appendix 3).
The TG AUC was stable over time (overall p: 0.55), while the TG lag times fell immediately after T0 (p<0.001 for all other time points vs. T0, after Bonferroni correction). The TG peak was lower at T0 than at T360 (p=0.002, after Bonferroni correction). The fibrin lysis time was stable over time (p=0.11). The PG AUC was higher at T360 than at T0 and T120 (p<0.001 and p=0.001 respectively, after Bonferroni correction). The PG peak time was longer at T0 than at all other time points (p<0.001 for all other time points vs. T0, after Bonferroni correction). The plasmin peak time was shorter at T60 than at T30 (p=0.045, after Bonferroni correction). The TG and PG peak times differed more at T0 than at all other time points except T360 (p<0.050 for all other time points vs. T0, after Bonferroni correction; Figure 3).
The results for the routine laboratory tests are summarized in Table 3. Fibrinogen and antithrombin levels fell soon after delivery (from T0 to T30: p=0.01 for both, after Bonferroni correction). The level of fibrin monomers increased from T0, reached a peak at T120 (p<0.001 for all times points; post-hoc p-values <0.05 after Bonferroni correction) and then fell at T360 (p=0.030 for T120 vs. T360, after Bonferroni correction). The concentration of TAT complexes changed in the same way, with an early increase from T0 at T30 and T60 and then a drop at T120 and T360. The D-dimers level increased later (from T30 to T60 and T120; p<0.001). Similarly, the level of PAP complexes increased from T0 to T30, T30 to T60, and T60 to T120 (p<0.001, p<0.001 and p=0.020, respectively, after Bonferroni correction).
Variables/Time/:n samples/comparisons
T0
n=37T30 n=37
T60 n=37
T120 n=37
T360 n=37
aOverall change vs. T0
bChange between T0 and each time point
cChange between time points
Hemoglobin (g/dL)
11.9 (1.1)
11.3 (1.2)
-
11.7 (1.1)
11.5 (1.3)
P=0.009
T0>T30 and T360 (p=0.007 and p=0.037 respectively)
No change
Platelet count (109/L)
227 (57)
203 (54)
-
217 (57)
223 (55)
P<0.001
T0>T30 and T120 (p<0.01 and p<0.002 respectively)
T30<T120 (p=0.018)
Fibrinogen (g/L)
4.9 (0.8)
4.6 (0.7)
-
4.7 (0.7)
4.5 (1.0)
P=0.003
T0>T30 and T360 (p=0.011 and p=0.002 respectively)
No change
Factor II (IU/ml)
122 (17)
120 (18)
-
122 (18)
117 (14)
P=0.16
No change
No change
Factor V (IU/ml)
107 (17)
106 (21)
-
112 (17)
106 (16)
P=0.43
No change
No change
Antithrombin (IU/ml)
95 (13)
88 (13)
-
90 (14)
92 (11)
P=0.001
T0>T30 and T120 (p<0.01 and p<0.010 respectively)
No change
Fibrin monomers (ng/L)*
12.5 [8 - 29.5]
35.0 [18.0 - 86.0]
-
645 [19.0-139.0]
23.0 [11.0 - 90.0]
P<0.001*
T0<all times (p<0.050)
T120>T360 (p=0.030)
D-dimers (μg/mL)*
1495 [1217 - 2220]
2710 [1910 - 3680]
-
3570 [2920 - 5750]
3890 [2733 - 5490]
P<0.001*
T0<all times (p<0.001)
T30<T120 (p<0.001)
Thrombin-antithrombin complexes (μg/L)*
26.9 [16.9 - 57.4]
59.3 [27.7 - 116.1]
81.1 [36.8 - 210.6]
29.4 [20.7 - 83.0]
14.2 [8.1 - 36.7]
P<0.001*
T0<T30 and T60 (p=0.030 and p<0.001 respectively)
T60>T120 (p=0.015) T120>T360 (p<0.001)
Plasmin-antiplasmin complexes (μg/mL)*
244.5 [204.0 - 299.0]
389.0 [302.0 - 487.0]
619.5 [461.0 - 1010]
811.0 [563.0 - 1336]
732.0 [610.0 - 1524]
p<0.001*
T0<all times (p<0.001)
T30<T60 (p<0.001) T60<T120 (p=0.02)
aOverall p-value in a linear mixed model for the time parameter as fixed effect. bP-values obtained after post-hoc analysis vs. T0, after Bonferroni correction. cP-values obtained after post-hoc analysis of consecutive time points, after Bonferroni correction. *non-normally distributed laboratory variables are expressed as the median [IQR], and p-values were obtained after rank transformation.
Table 3: Changes over time in laboratory data.
The associations between changes over time in the fibrinogen concentration, the D-dimer concentration and the SGTPA variables are summarized in Table 4. At all-time points, an increase in the fibrinogen concentration was associated with increases in the thrombin peak (estimated ES=152.5 (40.5); p<0.001), the thrombin lag time (estimated ES=0.65 (0.30); p=0.005), the thrombin peak time (estimated ES=0.77 (0.27); p=0.005) and the plasmin peak time (estimated ES, 1.49 (0.50); p=0.004). Likewise, a greater fibrinogen concentration was correlated with a lower fibrin lysis time at all-time points (estimated ES= -0.79 (0.28); p=0.007). At all-time points, an increase in the D-dimer level was associated with a decrease in the thrombin lag time (estimated ES= -0.27 (0.12); p=0.031) and the plasmin peak (estimated ES= -0.34 (0.14); p=0.018) and an increase in the fibrin lysis time (estimated ES= 0.33 (0.16); p=0.046). There were no significant associations between ideal bodyweight and SGTPA variables at T0. At T0, total blood loss was positively correlated with plasmin AUC (r=0.34, p=0.044) and negatively correlated with plasmin peak time (r= -0.60, p<0.0001) and the time interval between the TG and PG peak times (r= -0.41, p=0.001). An increase in additional blood loss was negatively correlated with the plasmin peak time (r= -0.44, p=0.006).
Estimates (SE)
p-value
Fibrinogen
Thrombin
Lag time
0.65 (0.30)
0.005
Peak
152.5 (40.5)
<0.001
Peak time
0.77 (0.27)
0.005
AUC
-311.4 (662)
0.64
Plasmin
Peak
0.25 (0.23)
0.27
Fibrin lysis time
-0.79 (0.28)
0.007
AUC
-137.7 (84.9)
0.11
Peak time
1.49 (0.50)
0.004
D-dimers
Thrombin
Lag time
-0.27 (0.12)
0.031
Peak
-0.20 (0.13)
0.12
Peak time
-0.24 (0.12)
0.057
AUC
-0.17 (0.10)
0.11
Plasmin
Peak
-0.34 (0.14)
0.018
Fibrin lysis time
0.33 (0.16)
0.046
AUC
-0.20 (0.11)
0.075
Peak time
-0.14 (0.13)
0.3
“Estimates” corresponds to the effect size for a one-point increment in the fibrinogen level or the D-dimer level in a mixed model. The fibrinogen and D-dimer levels were included in a linear mixed model as fixed effects over time, and a random effect was included to take account of the repeated measures per patient. The SGTPA variables were the explanatory variables.
Table 4: Associations between changes over time in fibrinogen and D-dimer levels and SGTPA variables.
Discussion
Summary of the findings
The results of the present study suggested that the SGTPA is valid and provides postpartum reference values for non-PPH CS. The baseline TG and PG were significantly greater in the non-PPG group than in non-pregnant women. In the non-PPH group, the TG and PG AUCs and the fibrin lysis time were remarkably stable over time. Plasmin generation increased (from 30 to 120 minutes after delivery) and was correlated with the parallel increase in D-Dimer and PAP complex levels. The plasmin peak time and the time interval between the thrombin and plasmin peaks were the most dispersed variables at T0 and, interestingly, were inversely correlated with the total physiological blood loss: the sooner the PG peaked and the shorter its lag after the thrombin peak, the greater the blood loss observed.
The SGTPA
In 2011, the NHA was validated as a tool for detect impairments in coagulation and fibrinolysis [12]. We developed an SGTPA on the basis of Van Geffen’s NHA, with a view to measuring TG and PG during PPH-induced coagulopathy [10]. The reference values for the immediate postpartum period highlighted an early increase in TG potential and a later increase in PG potential. The coefficients of variation for the immediate postpartum period were similar to the literature values, suggesting that the SGTPA is accurate during this specific period [11]. These initial results indicate that the SGTPA can be used to investigate the placental bed and the systemic activation of coagulation and fibrinolysis after delivery.
Fibrinolysis in the immediate postpartum period, relative to indirect markers
Fibrinolysis increases rapidly following a physiological delivery and expulsion of the placenta, resulting in elevated D-dimer levels [18,19]. In a previous RCT, D-dimers and PAP complexes were measured in a non-hemorrhagic group (n=23), an untreated Hemorrhagic group (H) (n=72), and a TA-Treated Hemorrhagic group (TA) (n=72) [6]. In the H group, the D-dimer level was significantly elevated at enrolment (3730 [2468-8493] ng.ml-1 vs. 1586ng.ml-1 [2267-4375] in the NH group; p=0.0001) and at +2h (7495 [4400-15772] ng.ml-1 vs. 2649ng.ml-1 [2267-4375], respectively; p=0.001) [6]. The PAP complex level at +30min (486μg.ml-1 [340- 1116] μg.ml-1) was significantly increased in the H compared to NH group [6]. Thrombin-Activatable Fibrinolysis Inhibitor (TAFI) levels do not change during pregnancy, fall after delivery, and are not correlated with D-dimer levels [25]. Levels of tissue-PA and its inhibitors increase over the course of pregnancy and then fall in the late postpartum period [26]. Lastly, viscoelastic tests have been used to detect fibrinolytic activity in the postpartum period [27-29]. However, the diagnosis of fibrinolysis is complicated by simultaneous early fibrinogenolysis that masks the fibrinolytic process [30,31].
Our present results are consistent with the changes over time in indirect markers of coagulation and fibrinolysis described in the literature. In our strictly selected population of women with non-PPH CS, the biomarkers of TG leading to fibrin formation changed soon after delivery (T0 to T30: a decrease in fibrinogen and antithrombin levels, and an increase in fibrin monomer and TAT complex levels). The increases in D-dimers and PAP complex levels started after T30.
Simultaneous TG and PG potential may provide a reliable direct marker in the immediate postpartum period
The SGTPA might be of value for studying interactions between coagulation components. Here, we introduced a new variable: the time difference between the TG peak and the PG peak. The fibrinogen level at T0 appears to markedly influence the TG-PG interaction: a higher fibrinogen level is associated with later TG and PG peaks, later fibrin lysis, and a greater the time interval between the TG and PG peaks [1,2]. Thrombin is expected to slow PG via the activation of TAFI. Activated TAFI masks the fibrin-lysine sites exposed on native fibrin and fibrin monomers [1,2]. SGTPA is influenced by the TAFI interaction, which constitutes an advantage of this assay [11]. The time difference between the TG peak and the PG peak might reflect this interaction. In fact, the PG potential was positively correlated with the D-dimer level. Lastly, PG potential was associated with total and additional blood losses, and blood loss was inversely correlated with the time to the PG peak. Thus, the SGTPA might provide more information on PPH-related fibrinolysis and the pharmacodynamic dose-ranging profile of TA than indirect biomarkers (such as D-dimers and PAP complexes) do [9,10].
Limitations
Firstly, our study only included patients undergoing elective CS. Thus, the observed changes might reflect the surgical activation of coagulation and fibrinolysis. However, the changes in hemostasis observed here were similar to those typically seen after normal vaginal deliveries, and tissue-factor-dependent blood coagulation is known to be enhanced after all types of delivery [32]. Moreover, the present study was an ancillary part of the TRACES pharmacodynamic study of the impact of low and standard doses of TA vs. placebo in hemorrhagic CS. Our objective was to establish the reference range for SGTPA variables after non-PHH CS.
Secondly, we studied a small number of non-pregnant women of childbearing age. However, we chose to make a rigorous comparison by limiting the central laboratory’s reference data to these individuals. The non-pregnant and non-PPH groups were compared using a nonparametric test.
Conclusion
SGTPA = simultaneous generation of thrombin and plasmin assay; non-PPH = non-hemorrhagic; TG=thrombin generation; PG = plasmin generation; CS=caesarean section, PPH = postpartum hemorrhage, TA = tranexamic acid.
Our study established a reference range for SGTPA variables after non-PHH CS. The SGTPA appears to be valuable for measuring TG and PG and the interaction between the two. The TG potential and coagulation activation increased immediately after delivery, whereas PG potential and fibrinolysis increased later. These post-CS reference values will be used to interpret PPH-induced changes and the impact of various dose levels of TA in the TRACES pharmacodynamic trial.
Declaration Section
Ethical approval and consent to participate
The TRACES trial (NCT0279711913, registered on June 13, 2016) was approved by the competent national bodies (ANSM: reference 201500249926) and an investigational review board (CPP Nord Ouest 15150; reference: 15/50 020216). All participants in the TRACES trial gave their written informed consent to participation in ancillary studies. In compliance with the French legislation on clinical trials (government order published on November 14, 2006), the study participants were registered in the French National Register of Clinical Trial Participants.
Consent for publication
The authors have read the present manuscript and given their consent for publication.
Availability of supporting data
Supporting data are available and presented in Appendices 1 to 3.
Funding
The TRACES trial was funded by grants from public-sector bodies: the French Ministry of Health (reference: PHRC N°14-0032) and the French National Agency for Medicines and Healthcare Products (Agence nationale de sécurité du médicament et des produits de santé, ANSM; reference: AAP-003).
Authors’ Contributions
Anne-Sophie Ducloy-Bouthors contributed to study conception and design, preliminary studies on specific laboratory test production, data acquisition, management, analysis and interpretation, and drafting and revision of the final version of the present manuscript submitted for publication. Fanny Lassalle contributed to revision of the manuscript. Sixtine Gilliot contributed to data analysis and interpretation, and drafting and revision of the final version of the manuscript. Maeva Kyheng contributed to data analysis and interpretation, and drafting and revision of the final version of the manuscript. Rémi Favier contributed to the data collection and analysis, and revision of the final version of the manuscript. Edith Peynaud contributed to the data collection and analysis, and revision of the final version of the manuscript. Claire Hémar contributed to the data collection and analysis, and revision of the final version of the manuscript. Benjamin Hennart contributed to study conception and design, data management, analysis, interpretation, and drafting and revision of the final version of the manuscript. Alexandre Turbelin contributed to data acquisition, management and analysis, and revision of the manuscript. Gilles Lebuffe contributed to data analysis, interpretation, and revision of the manuscript. Alain Duhamel contributed to study conception and design, data management, analysis, interpretation, and drafting and revision of the manuscript. Sophie Susen elaborated contributed to study conception and design, preliminary studies of the STPGA, data acquisition, management, analysis, interpretation, and drafting and revision of the final version of the manuscript. Emmanuelle Jeanpierre contributed to study conception and design, preliminary studies of the STPGA, data acquisition, management, analysis, interpretation, and drafting and revision of the final version of the manuscript. Pascal Odou contributed to the data analysis and interpretation, and revision of the final version of the manuscript. All authors read and approved the final manuscript.
Acknowledgement
We especially thank Christine Nobecourt, the TRACES research midwife who helped with study conception, elaboration of the sample logistics, elaboration of the electronic case report form, and data collection, management and quality control. Florence Duflot helped to develop the electronic case report form and contributed to data management and quality control. Rama Sambou contributed to data management and quality control. Imen Saidi, Anne-Sophie Baptiste, and Nawal Benzekri contributed to data acquisition and quality control. The TRACES study investigators Hawa Keita-Meyer, Agnès Rigouzzo and Laurent Chonow contributed to data acquisition. And Pr David M. Fraser, native English speaker (PhD in bioscience from the University of Oxford, a professional medical writer having drafted or copy-edited over 1100 manuscripts who copy-edited the manuscript).
References
- Longstaff C. Measuring fibrinolysis: from research to routine diagnostic assays. J Thromb Haemost. 2018; 16: 652-662.
- Kolev K, Longstaff C. Bleeding related to disturbed fibrinolysis. Br J Haematol. 2016; 175: 12-23.
- Ker K, Edwards P, Perel P, Shakur H. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative metanalysis. BMJ. 2012; 344: e3054.
- McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012; 72: 585-617.
- Ducloy-Bouthors A-S, Jude B, Duhamel A, et al. High-dose tranexamic acid reduces blood loss in postpartum haemorrhage. Crit. Care. 2011; 15: R117.
- Ducloy-Bouthors AS, Duhamel A, Kipnis E, et al. Postpartum haemorrhage related early increase in D-dimers is inhibited by tranexamic acid: haemostasis parameters of a randomized controlled open labelled trial. Br. J. Anaesth. 2016; 116: 641-648.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with postpartum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389: 2105-2116.
- Shakur-Still H, Roberts I, Fawole B, et al. Effect of tranexamic acid on coagulation and fibrinolysis in women with postpartum haemorrhage (WOMAN-ETAC): a single-centre, randomised, double-blind, placebocontrolled trial. Wellcome Open Res. 2018; 3: 100.
- Bouthors A-S, Hennart B, Jeanpierre E, et al. Therapeutic and pharmacobiological, dose-ranging multicentre trial to determine the optimal dose of TRAnexamic acid to reduce blood loss in haemorrhagic CESarean delivery (TRACES): study protocol for a randomised, double-blind, placebo-controlled trial. Trials. 2018; 19: 148.
- Ducloy-Bouthors A-S, Jeanpierre E, Saidi I, et al. TRAnexamic acid in hemorrhagic CESarean section (TRACES) randomized placebo controlled dose-ranging pharmacobiological ancillary trial: study protocol for a randomized controlled trial. Trials. 2018; 19: 149.
- Van Geffen M, Loof A, Lap P, et al. A novel hemostasis assay for the simultaneous measurement of coagulation and fibrinolysis. Hematol. Amst. Neth. 2011; 16: 327-336.
- Van Geffen M, Menegatti M, Loof A, et al. Retrospective evaluation of bleeding tendency and simultaneous thrombin and plasmin generation in patients with rare bleeding disorders. Haemophilia. 2012; 18: 630-638.
- Bagot CN, Leishman E, Onyiaodike CC, et al. Normal pregnancy is associated with an increase in thrombin generation from the very early stages of the first trimester. Thromb Res. 2017; 157: 49-54.
- Erez O, Gotsch F, Mazaki-Tovi S, et al. Evidence of maternal platelet activation, excessive thrombin generation, and high amniotic fluid tissue factor immunoreactivity and functional activity in patients with fetal death. J Matern Fetal Neonatal Med. 2009; 22: 672-687.
- Joly BS, Sudrié-Arnaud B, Barbay V, et al. Thrombin generation test as a marker for high risk venous thrombosis pregnancies. J. Thromb. Thrombolysis. 2018; 45: 114-121.
- Dallaku K, Shakur-Still H, Beaumont D, et al. No effect of tranexamic acid on platelet function and thrombin generation (ETAPlaT) in postpartum haemorrhage: a randomised placebo-controlled trial. Wellcome Open Res. 2019; 4: 21.
- Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost. 2003; 29: 125-130.
- Gerbasi FR, Bottoms S, Farag A, Mammen E. Increased intravascular coagulation associated with pregnancy. Obstet Gynecol. 1990; 75: 385-389.
- Cerneca F, Ricci G, Simeone R, Malisano M, Alberico S, Guaschino S. Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. Eur J Obstet Gynecol Reprod Biol. 1997; 73: 31-36.
- Coolman M, de Groot CJM, Steegers EAP, Geurts-Moespot A, Thomas CMG, Steegers-Theunissen RPM, et al. Concentrations of plasminogen activators and their inhibitors in blood preconceptionally, during and after pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006; 128: 22-28.
- Wright JG, Cooper P, Astedt B, Lecander I, Wilde JT, Preston FE, et al. Fibrinolysis during normal human pregnancy: complex inter-relationships between plasma levels of tissue plasminogen activator and inhibitors and the euglobulin clot lysis time. Br J Haematol. 1988; 69: 253-258.
- Bonnar J, Davidson JF, Pidgeon CF, McNicol GP, Douglas AS. Fibrin degradation products in normal and abnormal pregnancy and parturition. Br Med J. 1969; 3: 137-140.
- Roberts I, Shakur H, Fawole B, Kuti M, Olayemi O, Bello A, et al. Haematological and fibrinolytic status of Nigerian women with post-partum haemorrhage. BMC Pregnancy Childbirth. 2018; 18: 143.
- Robinson JD, Lupkiewicz SM, Palenik L, Lopez LM, Ariet M. Determination of ideal body weight for drug dosage calculations. Am J Hosp Pharm. 1983; 40: 1016-1019.
- Chabloz P, Reber G, Boehlen F, Hohlfeld P, de Moerloose P. TAFI antigen and D-dimer levels during normal pregnancy and at delivery. Br J Haematol. 2001; 115: 150-152.
- Bremme K, Ostlund E, Almqvist I, Heinonen K, Blombäck M. Enhanced thrombin generation and fibrinolytic activity in normal pregnancy and the puerperium. Obstet Gynecol. 1992; 80: 132-137.
- Faraoni D, Van der Linden P, Ducloy-Bouthors A-S, Goobie SM, DiNardo JA, Nielsen VG. Quantification of Fibrinolysis Using Velocity Curves Measured with Thromboelastometry in Children with Congenital Heart Disease. Anesth Analg. 2015; 121: 486-491.
- Arnolds DE, Scavone BM. Thromboelastographic Assessment of Fibrinolytic Activity in Postpartum Hemorrhage: A Retrospective Single-Center Observational Study. Anesth Analg. 2020; 131: 1373-1379.
- Collins NF, Bloor M, McDonnell NJ. Hyperfibrinolysis diagnosed by rotational thromboelastometry in a case of suspected amniotic fluid embolism. Int J Obstet Anesth. 2012.
- Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013; 11: 307-314.
- Collins PW, Lilley G, Bruynseels D, et al. Fibrin-based clot formation an early and rapidly available biomarker for progression of postpartum hemorrhage: a prospective cohort study. Blood. 2014; 124: 1727-1736.
- Hoffman M, Pawlinski R. Hemostasis: old system, new players, new directions. Thromb. Res. 2014; 133: S1-S2.
- Boer K, den Hollander IA, Meijers JCM, Levi M. Tissue factor-dependent blood coagulation is enhanced following delivery irrespective of the mode of delivery. J Thromb Haemost. 2007; 5: 2415-2420.
