Abstract
Acquired immune deficiencies caused by different etiologies, promote invasive fungal infections. When this immunity begins to improve, it can induce an excessive inflammatory response defined as Immune Reconstitution Inflammatory Syndrome (IRIS). Hepatosplenic Candidiasis (HSC) can be considered a form of IRIS syndrome as it occurs following neutrophil recovery in patients treated for acute leukemia. Differentiating IRIS from a single fungal infection or treatment failure due to a similar clinical picture is a real diagnostic problem. Misdiagnosis and subsequently ineffective treatment with antifungal therapy instead of anti-inflammatory drugs, may lead fatal course of the disease.
A deep and prolonged neutropenia developed after the first induction chemotherapy in our two and a half-year-old male patient who was followed up in our clinic with the diagnosis of Acute Myeloblastic Leukemia (AML). Our patient had fever, abdominal pain as well as his Gamma Glutamyl Transferase (GGT) and Alkaline Phosphatase (ALP) levels increased during neutropenia recovery. He was diagnosed with hepatosplenic candidiasis, by observing ‘target like abscesses’ on dynamic Magnetic Resonance Imaging (MRI) taken for his newly developing symptoms and laboratory findings while recovering neutropenia. After his first and third induction chemotherapy courses, his fever persisted although antifungal therapy, steroid treatment was initiated considering IRIS. After his re-intensification course, because of the same flare-up symptoms, we started immunglobulin in addition to steroid. With methylprednisolone and intravenous immunoglobulin, his symptoms improved and significant regression was observed in the lesions ‘target-like abscesses’ on MRI and in the laboratory values.
Result: IRIS should be considered for patients with hepatic candidiasis whose have persistent fever despite appropriate antifungal therapy. Glucocorticoid should be started first for an anti-inflammatory effect.
Keywords: Immune reconstitution inflammatory syndrome; Antiinflammatory cytokines; Hepatosplenic candidiasis; Interferon gamma; Proinflammatory cytokines
Introduction
Candida is a common patogen worlwide. It can contribute high mortality and morbidity to cancer patients by causing invasive candidiasis infection. Hepatosplenic Candidiasis (HSC), also called ‘chronic disseminated candidiasis’, is a part of severe invasive Candida infection occurs with distinct involvement in the liver, spleen, sometimes kidneys, and other organs. The incidence of HSC in patients with acute leukemia ranges from 2.0% to 7.4%. HSC is a unique clinical finding for invasive candidiasis. It occurs following neutrophil recovery after chemotherapy in patients with acute leukemia and effects organs as liver and spleen. The patogenesis of HSC is unknown. Studies suggest that Candida species colonized in the intestines owing to mucosal damage after chemotheraphy and by passing portosplenic circulation, they invade and proliferate to the hepatosplenic sinusoids [1].
Acquired immune deficiencies caused by different etiologies, including Human Immunodeficiency Viruse (HIV), antineoplastic drugs, immunosuppressive therapy used in solid organ recipients, immunomodulator drugs and other biologic agents, all encourage invasive fungal infections. Subsequent immune recovery induces excessive inflammatory response called Immune Reconstitution Inflammatory Syndrome (IRIS) that conduce significant morbidity and mortality in patients. Hepatosplenic Candidiasis (HSC) can be considered a form of IRIS syndrome as it occurs following neutrophil recovery in patients treated for acute leukemia. Differentiating IRIS from a single fungal infection or treatment failure due to a similar clinical picture is a real dilemma. Diagnostic faults following ineffective treatment with antifungal therapy instead of anti inflammatory drugs, may lead fatal course of the disease [2].
We aimed to present our case who developed HCS and IRIS after recovery from prolonged neutropenia and how we treated, due to its rarity.
Our aim is to present a case with HSC/IRIS who remained neutropenic for a long time after acute myeloblastic leukemia induction treatment and developed an excessive inflammatory response during neutrophil recovery.
Case Presentation
Our case is two and a half years old male patient. He was applied to another hospital for his fever, pain, swelling and redness behind the right ear. A postauricular mass was detected on Magnetic Resonance Imaging (MRI) and excisional biopsy performed. The biopsy result reported as ‘ALL or AML is considered first’. Although his family taken the report, they hadn’t been, apply any doctor for a while. Seven months after biopsy, he was admitted to our hospital with complaints of cough, fever, generalized pain and abdominal swelling.
On physical examination, he had pallor, jaundice, left orbital propitosis, massive hepatosplenomegaly, and right postauricular lymphadenopathy. Laboratory tests was performed. The patient’s leukocytes 9200/uL, neutrophiles 1120/uL, Hb 5.9g/dl, HCT 18%, MCV 82.1fl, thrombocytes 38.000/uL, CRP 4.17mg/dL, LDH 369U/L, Uric Acid 2.7mg/dL, GGT 350U/L, ALP 311U/L, ALT 79U/L, AST 38U/L, Urea 13mg/dL, Creatinine 0.47mg/dL, Na 132mEq/L, K 3.7mEq/L, Albumin 3.32g/L, Ca 7.7mg/dL, Total bilirubin 4.36mg/ dL, direct bilirubin 3.57mg/dL, Amylase 240U/L, Lipase 668U/L.
He had pansitopenia, and there were blasts on his peripheral blood smear. We performed bone marrow aspirate. Blasts were observed on his bone marrow aspiration smears. Also flow cytometry detected blasts at a range of 67% and blasts were positive for CD13, CD33, HLA-DR, and Myeloperoxidase. We diagnosed acute myeloid leukemia to your patient with these results. We started AML-BFM 2019 protocol. In his genetic results, the MLL gene rearrangement (46, XY INS (10:11) (p12; q23q13) [14]/46, XY [2]) was positive, so we considered the patient to be at High Risk (HR). After the initial induction chemotherapy (AIE), a complete molecular response was achieved (No blasts and 11q23 rearrangement detected on flow cytometry).
Magnetic resonance imaging of the head revealed an 18x13x15 mm enhancing a mass lesion (myeloid sarcoma) in the left orbital area settled in intraconal legion. In addition to there were two mass lesions (chloroma) which nearly obliterated both maxillary sinuses. After first induction therapy, all of lesions were disappeared on his MRI.
Significant filling defect was observed in the right transverse sinus and proximal to the sigmoid sinus in magnetic resonance venography, and the appearance was reported to be consistent with dural sinus vein thrombosis. Subcutaneous enoxaparin was started at a dosage of 200mg/kg/d (twice a day) for sinus vein thrombosis. After four days of enoxaparin administration, despite platelet transfusion, his platelets could not be increased above 50,000/mm3. Therefore, enoxaparin could not to be applied and port catheter could not to be inserted. Platelet refractoriness due to sinus vein thrombosis was considered. During therapy his sinus vein thrombosis was regressed on MRI venography, and his platelets levels rose up to 50,000/mm3. Enoxaparin was continued.
Severe neutropenia was occurred after first induction chemotherapy. The patient’s neutropenic situation continued approximately twenty days. Appropriate antibiotic therapy was used for fever in neutropenic period. While his neutrophil counts was rising up, fever was started intermittently. Because of fever and increased direct bilirubin, GGT and ALP levels, hepatic candidiasis was considered. In abdominal MRI revealed multiple cystic lesions that was hypointense in T1A series, and hyperintense in T2A series with heterogeneous internal structure, in the liver paranchyma. The largest lesion was 20x23 mm in size in the liver right lobe 8th segment. After intravenous contrast application, some lesions (abcesses) were showed enhancement in the form of peripheral rim (target-like) and not enhancing in the central part were observed. Cystic lesions in the spleen and both kidneys, mostly in the liver, showed diffusion restriction compatible with abscess. With these findings were diagnosed as hepatosplenic candidiasis (Figure 1). Amphotericin B was started to the patient at a 5mg/kg/d dosage. Ursodeoxycholic aside was added his therapy for intrahepatic cholestasis (30mg/kg/d). In his follow up; his fever continued, and Vancomycin Resistant Enterococcus Faecalis (VRE) was isolated from his blood culture. We started linezolid (Minimal inhibitory concentration (MIC): 2). Although treatment, his fever did not decrease and he got worse. Laboratory findings related infections and cholestasis had not declined. Linezolid was changed with tigecycline (MIC: 0.12). On physical examination, he had severe oral mucositis and abdominal tenderness, therefore his oral intake was discontinued and total parenteral nutrition was started. Hypoalbuminemia was occurred, supportive treatment was given as albumin infusions. Together with these treatment changes, he had got better and his fever had decreased. His laboratory markers had decreased also. Intermittent fever was started, and his GGT and ALP levels was increased again despite of healing for his physical findings and rising his neutrophil counts. We suspected that the patient had IRIS, and we started methyl prednisolone at a dosage of 1mg/kg/d for 1 week. The patient’s fever disappeared immediately, and methyl prednisolone was tapered and stopped at the end of the third week.
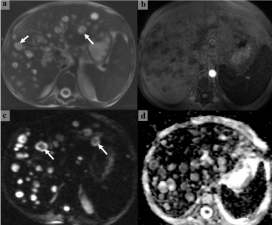
Figure 1: Target-like abscesses (bull’s-eye lesions) are observed in T2-
weighted (a), fat-suppressed T1-weighted (b), diffusion-weighted (c), and
ADC mapping (d) images obtained in the first magnetic resonance imaging
of the liver.
Our patient’s fever, abdominal pain occurs, and his leukocyte count (40.000/mm3), and crp levels increased again, while his neutrophil level that had dropped after the second induction chemotherapy (HAM), was rising. He had severe abdominal pain and his abdomen was distended. Abdominal X-Ray was performed, free subdiaphragmatic gas was detected. The patient operated with the diagnosis of intestinal perforation. Perforation and necrosis were observed in the ileum. Postoperative complications were not observed.
After the third chemotherapy cure (AI), the patient had intermittent fever over again and his GGT and ALP values increased when neutrophil recovery time. Methyl prednisolon was started at 1mg/kg/g for one week and stopped the third week by tapering. Meanwhile, he did not have a fever.
Abdominal ultrasonography was performed for the raise of the patient’s GGT and ALP levels, simultaneously fever, abdominal pain when his neutrophil count was increasing after the end of his fourth chemotherapy course (hAM). Mild dilatation (9mm) in the bile ducts in the left lobe of the liver, calculus and sludge in the bile duct were revealed on the abdominal ultrasonography. In his blood culture, streptococcus parasanguis and staphylocuccus epidermidis were isolated at the same time. Teicoplanin was added to his treatment that he was currently taking meropenem for fever, and Amphotericin B for candidiasis. However, the patient’s fever continued, and his laboratory values rose again (Crp, ALP, GGT, direct bilirubin levels). IRIS and intrahepatic cholestasis due to inflammatory response in liver considered and we added methyl prednisolon and ursodeoxycholic acid in his treatment. However, this time, there was no improvement in the clinical and laboratory findings of the patient. We suggested that the medications used for him could be the reason of his intrahepatic cholestasis. Steroid, meropenem, and amphotericin B were stopped. Levofloxacin and fluconazole were started. Upon the continuation of his fever, treatment of teicoplanin and fluconazole were changed as vancomycin and voriconazole. Intravenous Immunoglobulin (IVIG) (1g/kg/d) was given to the patient. Then his laboratory and clinical findings improved rapidly. Significant regression in size and numbers of hepatic and splenic lesions was observed on MRI findings in the third month of treatment, compared with initial scanning (Figure 2). The patient who has HLA full-matched sibling, transferred to another center for hematopoietic stem cell transplantation.
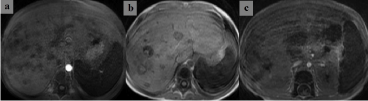
Figure 2: Significant morphological regression (change) of the lesions is observed in T1-weighted sections taken from the liver in the second and third months of
antifungal therapy.
Discussion
The incidence of opportunistic infectious diseases, including fungi, has increased tremendously due to the increased prevalence of acquired immune deficiencies following improved medical advances such as biological, immonomodulatory and intensive antineoplastic chemotherapy agents, increased use of hematopoietic stem cells, and solid organ transplantation. Further advances such as Antiretroviral Therapy (ART) in HIV patients managed to restore immunity. This new immune situation has defined as a new syndrome: the Immune Reconstitution Inflammatory Syndrome (IRIS). IRIS is currently known to occur during the course of various invasive fungal infections. This syndrome is defined as emerging infection or clinical worsening of a known infection disease after immune recovery. This reversal can be triggered by the use of ART in HIV patients, neutrophil increasing after chemotherapy and/or stem cell transplantation, poor stabilization of immunosuppressive therapy after solid organ transplantation, and even immunological changes of postpartum period. IRIS start by the recovery of immune cells, resulting in a ‘cytokine storm’ and an extreme host inflammatory response. This syndrome has been best described in HIV-infected patients treated with ART. In these patients; IRIS has been observed in the first six months of therapy and associated with variety of opportunistic infections caused by JC virus, mycobacterium tuberculosis, cytomegalovirus, cryptococcocci, and histoplasma species [2].
Hepatic candidiasis, also called ‘Chronic Disseminated Candidiasis’ (CDC) is a condition suspected of IRIS related candidiasis. This clinical condition develops in patients who have recently experienced profound and prolonged neutropenia, particularly neutrophil recovery after chemotherapy for acute leukemia. Diagnosis is usually made within 2 weeks of immune recovery [3]. Symptoms as fever and abdominal pain usually continue despite of antifungal treatment [2]. HSC is defined as multiple, small, peripheral targetlike abscesses (bull’s-eye lesions) in the liver or spleen showed on Computerized Tomography (CT), MRI or, ultrasound imaging. It is accompanied by high levels of Gamma Glutamyl Transferases (GGT) and alkaline phosphatase. Supporting microbiological properties is not required to meet the European Organization for Cancer Research and Treatment (EORTC) criteria for HSC [1].
In our patient, symptoms (fever and abdominal pain) and high levels of GGT and ALP were observed within two weeks following the immune recovery after a severe neutropenia lesting for 20 days. On MRI; multiple target-like abscesses (bull’s-eye lesions) were detected mostly in the liver parenchyma, in the spleen, and in the kidney, then the diagnosis was made. MRI is more sensitivity than ultrasonography and CT for detecting micro-abscesses that are most often localized in the liver and spleen and resulting from an excessive inflammatory response [4]. Using Positron Emission Tomography (PET) for diagnosis of HCS can shows promising results [5].
Innate immune system cells such as monocytes, macrophages and neutrophils are draw attention in the pathophysiology of IRIS. Because granulomas are often found in IRIS lesions [3]. Indeed, granulomas are the hallmark of histopathology in chronic disseminated candidiasis and they are commonly found in the IRIS related to the other fungal infections [3,6]. Interferon gamma (IFN-γ) that is produced excessively by T Helper-1 (Th1), neutrophils, or, macrophage cells, stimulates the formation of granulomas by activates fagositic activity of macrophages and also causes differentiations macrophages from monocytes [7].
Inadequate balance between proinflammatory Th1 response and antiinflammatory Th2 response is usually accepted as source of IRIS. Recently discovering the response of Th17 and regulator T cells (Treg), this model transformed as imbalance between proinflammatory Th1/ Th17 axis and antiinflammatory Th2/Treg axis [8-11]. Th1 and Th17 cells are proinflammatory cells and they produce IFN-γ that stimulates activation and differentiation of macrophages to M1 macrophages. M1 macrophages support for formation of granulomas and they produce more IFN-γ, thus creating an amplification loop that leads to an explosion of inflammation. As a result, IRIS is believed to be caused by an unregulated Th1/Th17 leading to increased interferon-γ (IFNγ) production [8].
Most of cases, epithelioid granulomas and micro-abscesses are seen at histopathological level. Blood cultures are negative in more than 80% of cases and microscopy reveals the presence of yeast in less than 50% of cases [3,12]. Although there were many micro abscesses in our case, no fungal growth was detected in blood cultures. While neutrophil values improve after each course of chemotherapy, the emergence of symptoms in the patient and the increase in GGT, ALP and CRP values may be mainly due to the proinflammatory cytokine interferon-γ (IFNγ) produced in the granulomas in the liver. Because granulomas consist of proinflammatory cells such as macrophages (M1), neutrophils, Th1 and Th17 cells.
An optimal duration of treatment for patients in hepatosplenic candidiasis has not been determined. However, long-term treatment is usually needed for weeks or months, even for one year. Treatment should be continued until all lesions are reliably cleared or fully healed. Antifungal therapy should also be individualized according to drug selection. Therefore, liposomal amphotericin B should be considered 2.5-5 mg/kg/day by IV infusion, at least in the first 6-8 weeks of treatment. Alternatively, fluconazole, itraconazole, and voriconazole can be used [13]. Although we used for our patient fluconazole/caspofungin when he was neutropenic, after developing HSC, fluconazole/caspofungin was discontinued and liposomal amphotericin B was started. In control, MRI performed four weeks later, a regression in the size and number of lesions in the liver was detected. In our patient who developed cholestasis after the fourth course of chemotherapy, we thought that cholestasis might be due to drugs, amphotericin was discontinued and voriconazole was started.
Hepatosplenic candidiasis occurs following neutrophil recovery in patients treated for acute leukemia, it has been suggested that this condition may represent a form of Immune Reconstitution Inflammatory Syndrome (IRIS) [14]. In the case of Hepatosplenic Candidiasis (HSC), persistent high fever, which is the only symptom in patients, is common despite appropriate antifungal therapy. There are many reports of patients with persistent fever and HSC in which adjuvant glucocorticoid therapy leads to rapid resolution of systemic symptoms [14-16]. In patients with persistent fever despite appropriate antifungal therapy, it might make sense to add lowdose glucocorticoids (0.5-1 mg/kg orally per day) to the antifungal regimen for several weeks (1-2 weeks) [13]. Because glucocorticoids are the only effective drug class now for treatment of IRIS [17]. They exert anti-inflammatory effects on most immune cells by altering the transcription of inflammatory mediators, interfering with nuclear factor-κB, and directly enhancing the action of anti-inflammatory proteins [18]. Briefly, glucocorticoids decrease Th1 cells response, but also they increase Th2 cells and Treg cells. Our patient had only fever after started antifungal treatment (amphotericin B). Our patient had intermittent high fever, high levels of GGT and ALP during the period of neutrophil recovery after the first and third chemotherapy courses, and we started him methyl prednisolon at a 1mg/kg/d dosage with diagnosed IRIS. With steroid treatment, our patient’s fever and laboratory findings were regressed.
Differentiating IRIS from a single fungal infection or treatment failure due to a similar clinical picture is a real diagnostic problem. Misdiagnosis and consequently ineffective treatment with antifungals instead of anti-inflammatory drugs may lead to a fatal course of the disease [2]. For this reason, we could not completely distinguish infection and IRIS, so we gave our patient intravenous immunglobulin at 1g/kg/d. The increases in the mentioned laboratory levels regressed rapidly and his symptoms (fever and abdominal pain) disappeared. Intravenous immunoglobulins have been used successfully in virusassociated IRIS; however, it has never been used in fungal-associated IRIS [2]. Therefore, we report the use of IVIG for the first time in a patient with hepatosplenic candidiasis, whose symptoms and laboratory findings did not improve despite the use of multiple antibiotics, antifungal, and steroids after the fourth chemotherapy (hAM) course and we thought that is beneficial.
Despite the platelet transfusion in the patient with high D-dimer levels and thrombosis, the platelet values could not be increased sufficiently. The cause of thrombosis may be blocking venous return as a cause of mass lesions in the maxillary sinus and in the left orbita. In MRI control after first induction, there were significant regression in these mass lesions, as well as regression in the sinus thrombosis in MRI venography. Because of the regression in these lesions, increases in thrombocyte values were detected after thrombocyte transfusion. The increase in platelet values can be explained by the removal of the venous return barrier due to compression of the tumor mass and the treatment of thrombosis.
Conclusion
Hepatosplenic Candidiasis (HSC) occurs following neutrophil recovery in patients treated for acute leukemia, this condition may represent a form of Immune Reconstitution Inflammatory Syndrome (IRIS). Differentiating IRIS from a single fungal infection or treatment failure/failure due to a similar clinical picture is a real diagnostic problem. Misdiagnosis and consequently ineffective treatment with antifungals instead of anti-inflammatory drugs can lead to a fatal course of the disease.
Results
As a result, in the case of Hepatosplenic Candidiasis (HSC) whose only symptom is persistent high fever despite appropriate antifungal therapy, IRIS should be considered and treated with glucocorticoids and IVIG as an anti-inflammatory treatment.
References
- Chen CY, Cheng A, Tien FM, Lee PC, Tien HF, Wang-Huei Sheng, et al. Chronic disseminated candidiasis manifesting as hepatosplenic abscesses among patients with hematological malignancies. BMC Infect Dis. 2019; 19: 635.
- Dellière S, Guery R, Candon S, Rammaert B, Aguilar C, Lanternier F, et al. Understanding Pathogenesis and Care Challenges of Immune Reconstitution Inflammatory Syndrome in Fungal Infections. J Fungi (Basel). 2018; 4: 139.
- Rammaert B, Desjardins A, Lortholary O. New insights into hepatosplenic candidosis, a manifestation of chronic disseminated candidosis. Mycoses. 2012; 55: e74-84.
- Anttila VJ, Lamminen AE, Bondestam S, Korhola O, Färkkilä M, Sivonen A, et al. Magnetic resonance imaging is superior to computed tomography and ultrasonography in imaging infectious liver foci in acute leukaemia. Eur J Haematol. 1996; 56: 82-87.
- Hot A, Maunoury C, Poiree S, Lanternier F, Viard JP, Loulergue P, et al. Diagnostic contribution of positron emission tomography with (18F) fluorodeoxyglucose for invasive fungal infections. Clin. Microbiol Infect. 2011; 17: 409-417.
- Kim HW, Heo JY, Lee YM, Kim SJ, Jeong HW. Unmasking Granulomatous Pneumocystis jirovecii Pneumonia with Nodular Opacity in an HIV-Infected Patient after Initiation of Antiretroviral Therapy. Yonsei Med J. 2016; 57: 1042-1046.
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000 Prime Rep. 2014; 6: 13.
- Sun HY, Singh N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis. 2009; 22: 394-402.
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008; 453: 1051-1057.
- Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: A (co-) evolutionary perspective. Nat. Rev. Immunol. 2009; 9: 883-889.
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T cell Lineages. Ann. Rev. Immunol. 2007; 25: 821-852.
- De Castro N, Mazoyer E, Porcher R, Raffoux E, Suarez F, Ribaud P, et al. Hepatosplenic candidiasis in the era of new antifungal drugs: a study in Paris 2000-2007. Clin Microbiol Infect. 2012; 18: E185-187.
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016; 62: e1-e50.
- Legrand F, Lecuit M, Dupont B, Bellaton E, Huerre M, Rohrlich PS, et al. Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis. 2008; 46: 696-702.
- Chaussade H, Bastides F, Lissandre S, Blouin P, Bailly E, Chandenier J, et al. Usefulness of corticosteroid therapy during chronic disseminated candidiasis: case reports and literature review. J Antimicrob Chemother. 2012; 67: 1493- 1495.
- Bayram C, Fettah A, Yarali N, Kara A, Azik FM, Tavil B, et al. Adjuvant Corticosteroid Therapy in Hepatosplenic Candidiasis-Related Iris. Mediterr J Hematol Infect Dis. 2012; 4: e2012018.
- Meintjes G, Scriven J, Marais S. Management of the Immune Reconstitution Inflammatory Syndrome. Curr HIV/AIDS Rep. 2012; 9: 238-250.
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005; 353: 1711-1723.
