Abstract
Background: Pure Red Cell Aplasia (PRCA) is a rare complication of ABO mismatched hematopoietic stem cell transplantation; there isn’t no standard of care, here we report a case of successful treatment by Rituximab in a refractory PRCA and chronic graft versus host disease.
Case Presentation: A 26-year-old woman with PRCA following ABOmismatched allogeneic HSCT for chronic myeloid leukemia, associated with steroid refractory chronic hepatic graft versus host disease, treated with 4 doses of Rituximab 375mg/m² weekly, with an increase in her hemoglobin level and improvement of her liver’s enzymes.
Conclusion: The interest of this case is to report the important therapeutic result of Rituximab, widely used in literature, especially if chronic Graft Versus host disease is associated.
Keywords: Pure red cell anemia; ABO mismatched hematopoetic stem cell transplantation; Chronic graft versus host disease; Rituximab; Steroids
Abbreviations
PRCA: Pure Red Cell Aplasia; cGVHd: Chronic Graft Versus Host Disease; HSCT: Hematopoietic Stem Cell Transplantation; CML: Chronic Myloid Leukemia; PBSCT: Peripheral Blood Stem Cell Transplantation; TKI: Tyrosine Kinase Inhibitors; DNA: Deoxyribonucleic Acid; EPO: Erythropoetin; CsA: Ciclosporin; CMV: Cytomegalovirus; PCR: Polymerase Chain Reaction
Background
The Human Leukocyte Antigen (HLA) system is independent from the blood group system, Incompatibilty ABO is not supposed to be an obstacle to allogeneic Hematopoietic Stem Cell Transplantation (HSCT), approximately 50% of HSCT are ABO incompatible. Different effects on engraftment have been observed after ABO mismatched HSCT: like a delayed red blood cell recovery and pure red cell aplasia [1,2].
Pure Red Cell Aplasia (PRCA) occurs in 29% after major ABO mismatched HSCT [1], it is explicated by the presence of recipient isoagglutinins against ABH antigens on donor Many risk factors for the development of PRCA have been identified.
The management of PRCA is not standardized, and several immunosuppressif treatments was reported Chronic Graft-Versus- Host Disease (GVHD) is a serious complication after hematopoietic stem cell transplantation, first line treatment is based on systemic corticosteroids but Various agents can be used for salvage therapy after corticosteroid failure, The correlation between chronic GVHD and PRCA post ABO mismatched transplantation and is not clear [1], in some cases the evolution of chronic GVHD lead to a spantaneous resolution of PRCA by the graft-versus-plasma cell effect [3].
Case Presentation
A 26-year-old woman with chronic phase of CML resistant to Thyrosine Kinase Inhibitors (TKI), with a T315I mutation, she underwent allogeneic PBSCT from an HLA-matched brother. There was major ABO incompatibility between the patient (O, RH+) and donor (B, RH+). A myeloablatif conditioning (Busilvex, Endoxan) was used with cyclosporin A 3mg/kg i.e. and short course methotrexate as GVHD prophylaxis.
Granulocytes exceeded 0.5×109/l on day 14 after PBSCT, and platelets exceeded 50×109/l on day 19. The hemoglobin level was stable around 11g/dl on day 14 without transfusion. Early ocular acute GVHD grade II was observed requiring low-dose steroid therapy + ciclosporine, thereafter her hemogolbin level fell rapidly to a nadir of 5 g/dl, 6g/dl on day 155, Absolute reticulocyte counts was 0.26x106/ mm³, There was no history of blood loss. Red cell autoantibodies were negative. Direct antiglobulin testing for IgG and complement was negative, Vitamin B12, folate were normal and ferritin levels was elevated, DNA analysis for the detection of parvovirus B19 in peripheral blood by polymerase chain reaction was negative. These findings led to a diagnosis of PRCA.
The patient was given EPO at a dose of 30000 IU s.c. weekly between days 170 and 200. Because of lack of efficacy, EPO was discontinued. On day 240 we begin to taper the CsA progressively and the patient started to have a liver dysfunction, a hepatic biopsy was compatible with chronic GVHD, we started prednisolone (PSL) 1mg/ kg/day p. o from day 240 to day 368, This therapy resulted in increased of hemoglobin level with no need RBC support and improvement in the chronic GVHD. Furthermore, the patient showed a rebound of her liver enzymes and decreased hemogolbin level after steroids withdrawal, We started Rituximab 375mg/m² once weekly for four weeks, hemoglobin and reticulocyte began to rise from 6.7g/dl to 12g/dl (day 503), the liver’s enzymes decreased with a normal level on day 658 (Figure 1). Otherwise on day 550 (2 months after first injection of Rituximab) the patient had shown a choriretinitis with a positive CMV PCR, we started an antiviral therapy by Ganciclovir iv 10mg/kg/day for 3 week followed by maintenance therapy for 2 week without an improvement then we switched to intraocular injections of Ganciclovir with a stability of retinal injuries.
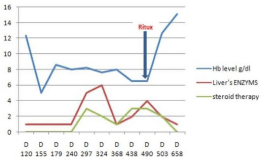
Figure 1: Clinical course and change of hemoglobin, Liver’s enzymes before
and after Steroids and Rituximab therapy.
After one year of follow up post Rituximab therapy, her hemoglobin level is stable around 15g/dl with normal liver function.
Discussion and Conclusion
Several cases of PRCA were reported following allogenic HSCT, mostly in the situations of major ABO incompatibility between recipient and donor like our case, but it is also described in one case after ABO matched allogenic HSCT [4]. The use of a reducedintensity conditioning [5], sibling donors [6] or the absence of acute graft-versus host disease [6] are also identified as a risk factor of PRCA, In a review of literature, the Major ABO-incompatibility was the most common risk factor found in 120 patients, The roles of reduced intensity conditioning cells is controversial, and same for the use or not of Methotrexate in the prophylaxis of GVHD [7].
In a retrospective study of a total of 153 patients [7], reduction of anti-donor isoagglutinin by in vivo adsorption, plasmapheresis or a combination of both methods had allowed a decrease of PRCA incidence (3% patients who had undergone anti-donor isoagglutinin reduction and in 16% who had not).
Management of PRCA after mismatched ABO incompatibility is not codified, a first option is a reduction of immunosuppression to enhance the graft-versus-plasma cell effects [3,4] but in our case it wasn’t possible because of active chronic liver GVHD ,Other treatments are available but they are only evaluated in small numbers series.
Our patient had developed PRCA and hepatic GVHD; both were refractory to steroids and Ciclosporin. Treatment with erythropoietin did not improve hemoglobin level.
In our knowledge the use of Rituximab in treatment of PRCA after ABO mismatched HSCT was reported in 5 cases with a good response (Table 1).
Patient Number
Protocol
Results
Our case
1
375mg/m2×4
Complete Remission
Maschan AA and et al. BMT. 2002 [8]
1
200mg/m2
Complete Remission
Helbig G and et al. Haematologica. 2005 [9]
2
150mg/m2
Complete Remission
DM Benson Jr and et al. BMT. 2008 [10]
1
375mg/m2×4
Complete Remission
Sung-Hoon Jung and et al. Case Rep Oncol 2012 [11]
1
375mg/m2×4
Complete Remission
Table 1: Results of Rituximab therapy for PRCA after ABO mismached incompatibility Allogenic HSCT.
Based on the implication of B cell in the pathogenesis of GVH, a Rituximab was evaluated in multiple case series [12-15] for treatment of refractory chronic GVHD and seems be effective.
Treatment by Rituximab in our case was very effective on both PRCA and GVHD, but was complicated by a CMV chorioretinitis. In a study of 46 patients, seventeen had received Rituximab before HSCT, Post-transplant infectious complications was present in 17.6% of them (CMV infection and CMV disease), and Twenty-nine of 46 patients without Rituximab treatment before HSCT did not develop CMV after HSCT [16]. Then the choice of this option should be balanced with its toxicity.
In Summary PRCA post, mismatched ABO HSCT is a serious complication and his treatment is not codified, as for cGVHD, we report this case to enrich the experience with Rituximab as a potential therapy for cGVHD and PRCA, it may be a promising treatment for such patients, particularly for those with steroid-refractory disease.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgement
I would like to thank the entire medical and paramedical team of the clinical hematology department of the Military hospital Mohammed V in Rabat, for the enormous work they do.
References
- Nina Worel. ABO-Mismatched Allogeneic Hematopoietic Stem Cell Transplantation. Transfus Med Hemother. 2016; 43: 3-12.
- Hirokawa M, Fukuda T. Efficacy and long-term outcome of treatment for pure red cell aplasia after allogeneic stem cell transplantation from major ABOincompatible donors. Biology of blood and marrow transplantation. 2013.
- Mielcarek M, Leisenring W, et al. Graft-versus-host disease and donordirected hemagglutinin titers after ABO-mismatched related and unrelated marrow allografts: evidence for a graft-versus-plasma cell effect Blood. 2000; 96: 1150-1156.
- Grigg AP and Juneja SK. Pure red cell aplasia with the onset of graft versus host disease. Bone Marrow Transplantation. 2003; 32: 1099-1101.
- Bolan CD, Leitman SF, Griffith LM, et al. Delayed donor red cell chimerism and pure red cell aplasia following major ABO-incompatible nonmyeloablative hematopoietic stem cell transplantation. Blood. 2001; 98: 1687-1694.
- Mielcarek M, Leisenring W, Torok-Storb B and Storb R. Graft-versus-host disease and donor-directed hemagglutinin titers after ABO-mismatched related and unrelated marrow allografts: Evidence for a graft-versus-plasma cell effect. Blood. 2000; 96: 1150-1156.
- Stussi G and et al. Prevention of pure red cell aplasia after major or bidirectional ABO blood group incompatible hematopoietic stem cell transplantation by pretransplant reduction of host anti-donor isoagglutinin, Ferrata Storti Foundation. 2009.
- Maschan AA, Skorobogatova EV, Balashov DN, et al. Successful treatment of pure red cell aplasia with a single dose of rituximab in a child after major ABO incompatible peripheral blood allogeneic stem cell transplantation for acquired aplastic anemia. Bone Marrow Transplant. 2002; 30: 405-407.
- Helbig G, et al. Successful treatment of pure red cell aplasia with repeated, low doses of rituximab in two patients after ABO-incompatible allogeneic haematopoietic stem cell transplantation for acute myeloid leukaemia. Haematologica. 2005.
- DM Benson Jr and et al. Successful therapy of chronic graft-versus-host disease manifesting as pure red cell aplasia with single-agent rituximab. Bone Marrow Transplantation. 2008; 41: 595-596.
- Sung-Hoon Jung and et al. Successful Treatment of Pure Red Cell Aplasia with Rituximab in Patients after ABO-Compatible Allogeneic Hematopoietic Stem Cell Transplantation. Case Rep Oncol. 2012; 5: 110-113.
- Cutler C and et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006.
- Ratanatharathorn V, Carson E, Reynolds C, et al. Anti-CD20 chimeric monoclonal antibody treatment of refractory immune-mediated thrombocytopenia in a patient with chronic graft-versus-host disease. Ann Intern Med. 2000; 133: 275-279.
- Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graftversus- host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003; 9: 505-511.
- Okamoto M, Okano A, Akamatsu S, et al. Rituximab is effective for steroidrefractory sclerodermatous chronic graft-versus-host disease. Leukemia. 2006; 20: 172-173.
- Lee MY, Chiou TJ, Hsiao LT, Yang MH, Lin PC, Poh SB, et al. Rituximab therapy increased post-transplant cytomegalovirus complications in Non- Hodgkin’s lymphoma patients receiving autologous hematopoietic stem cell transplantation. Ann Hematol. 2008; 87: 285-289.
