Abstract
Introduction: In Tunisia, the main etiology of cirrhosis remains viral hepatitis. Hepatocellular Carcinoma (HCC) is a major cause of mortality during cirrhosis. Its prognosis depends on several factors. The aim of this study was to compare HCC occurring on viral and non-viral cirrhosis and to determine the impact of the etiology of cirrhosis on the prognosis and survival of HCC.
Patients and Methods: A comparative retrospective study including 93 patients (January 2002 - December 2016) was performed. Patients were divided into two groups according to the etiology of their cirrhosis: group 1: patients with HCC du to viral cirrhosis and group 2: patients with HCC due to non-viral cirrhosis. Epidemiological, clinical, biological, radiological, therapeutic parameters as well as survival were compared between the two groups.
Results: Ninety-three patients with HCC (mean age 66.18 years (43-90 years) and sex ratio 1.66) were included. Cirrhosis was viral (group 1) in 68 (73%) and non-viral (group 2) in 25 (27%) patients. There were no differences in age, sex, history, habits or clinical signs. However, HCC was more frequently discovered during screening in group 1 (p=0.001). A higher level of GGT and PAL was observed in group 1 (p respectively=0.021 and 0.004). Moreover, higher tumoral size of HCC was noted in group 1 (45mm versus 31mm with p ‹0.0001). OKUDA and BCLC scores were higher in group 1 (p=0.018 and 0.020). Symptomatic treatment was more frequently indicated in group 1 (p=0.021). Finally, mean survival in both groups was respectively 4 months and 10 months (p=0.011) with a higher survival at 6 and 18-month in group 2 (p=0.047 and 0.05).
Conclusion: In our study, viral cirrhosis was correlated with more advanced HCC as evidenced by higher tumoral size and elevated prognostic scores. Survival was thus lower in the group of viral cirrhosis. With the recent development of anti-viral therapies, the impact of the etiological treatment of cirrhosis on the evolution of HCC should be assessed.
Keywords: Hepatocellular Carcinoma; Prognosis; Hepatitis
Introduction
Cirrhosis caused by Hepatitis B Virus (HVB) or Hepatitis C Virus (HCV) [1] are responsible for the majority of cases of Hepatocellular Carcinoma (HCC) [2]. The etiology of cirrhosis appears than to influence phenotypic and evolutive characteristics, as well as the prognosis and survival during HCC.
The aim of our study was to compare HCC occurring in viral and non-viral cirrhosis and to assess the impact of the etiology of cirrhosis on prognosis and survival during HCC.
Patients and Methods
A retrospective analytical study including all HCC on hepatic cirrhosis was conducted. Patients were followed in the gastroenterology department of Habib Thameur Hospital during a period of 15 years (January 2002 - December 2016).
Patients were divided into two groups according to the etiology of their cirrhosis:
• Group 1: patients with HCC on viral cirrhosis (due to hepatitis B or C).
• Group 2: patients with a HCC on cirrhosis of other etiology.
Cirrhosis was diagnosed on clinical, biological (prothrombin time, platelets, albumin and fibrotic markers), endoscopic (oesophageal varices), and morphological signs.
The diagnosis of HCC, due to the evolution of the diagnostic criteria, was made, according to the date of diagnosis, on the Barcelona criteria of 2000 [3], the AASLD (American Association for The Study of Liver Diseases) criteria of 2005 [4] and 2011 [5] and finally, on the EASL (European Association for The Study of Liver) criteria of 2012 [6].
Patients with HCC on non-cirrhotic liver (healthy liver or chronic hepatitis) and patients whose hospital records were either unexploitable or not found were not included in our study.
Patients whose follow-up was less than 6 months were excluded from the survival study.
Epidemiological, clinical, biological, morphological, therapeutic and outcome data, as well as survival, were collected and compared between the two groups.
Statistical analysis was done by SPSS 23.0 software. Comparison of qualitative variables was performed by the Pearson Chi 2 test. Comparison of quantitative variables was performed by Student’s t-test. Survival was assessed according to the Kaplan-Meier method with comparison of survival rates by the Log Rank test.
In all statistical tests, the significance level was set at 0.05.
Results
Descriptive study
During the study period, 105 patients with HCC were recorded. Twelve patients were excluded: four patients had HCC on healthy liver and two on chronic hepatitis. The remaining 6 patients were excluded due to missing data or unusable records. Thus, only 93 cases were included (Figure 1).
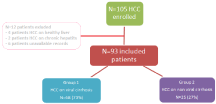
Figure 1: Repartition of the groups according to the etiology of cirrhosis.
Mean age was 66.18 years [43-90 years] and sex ratio was 1.66.
Cirrhosis was due to hepatitis C virus in 44% of cases (N=41), to hepatitis B virus in 29% of cases (N=27). Group 1 included than 68 patients (73%). Group 2 including cirrhosis of non-viral etiology included 25 patients (27%): 8 cases of alcoholic cirrhosis, 3 cases of primary biliary cirrhosis, 2 cases of nonalcoholic steatohepatitis and 1 case of hepatitis autoimmune. In 9 cases, cirrhosis was of undetermined etiology despite an exhaustive etiological assessment.
Cirrhosis was classified Child Pugh A, B and C in respectively 31.2%, 47.3% and 21.5% of patients.
Upper endoscopy found oesophageal varices in 79 cases (86%), gastric varices in 9 patients (9.8%) and hypertensive gastropathy in 37 patients (40.2%).
HCC was diagnosed after an average delay of 18.13 months [0- 240 months].
Alphafoetoprotein level was high in 69 patients with a mean level of 22262.84 ng/ml [2-877000 ng/ml]
Portal invasion was objectified in 32.9% of cases (N=24). Also, thrombosis of the spleno-mesareic venous trunk, superior mesenteric vein or inferior vena cava was found in 5 cases.
Metastases were detected in 9.7% of patients (N=9): five cases of lung metastases, a case of bone metastases, a case of splenic metastases, a case of surrenal metastases and finally a case of brain metastases.
According to MILAN criteria:
• Small HCC group (single nodule of less than 5 cm or a maximum of 3 nodules that do not exceed 3 cm each without vascular invasion, lymph node or metastases) included 27 patients (29%).
• Advanced HCC Group (HCC who does not meet the above-mentioned criteria) included 66 patients (71%).
• Performance status was stage 0 in 55% (N=51) stage 1 or 2 in 39.7% (N=37) and stage 3 or 4 in 5.3% (N=5).
The OKUDA score and the BCLC are resumed in Figure 2.
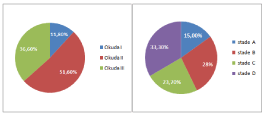
Figure 2: OKUDA and BCLC classification.
Concerning the management of HCC, curative treatment was proposed for 12 patients (12.9%) (2 cases of hepatic resection and 10 cases of percutaneous destruction (including 8 cases of radiofrequency and 2 cases of alcoholic desctruction)).
Palliative treatment was indicated in 17 patients (18.3%): 14 patients had intra-arterial embolization chemotherapy while antiangiogenic treatment with sorafenibwas prposed in 3 patients.
The rest of the patients (68.8%, N=64) had symptomatic treatment.
Mean follow-up was 11.35 months [6-84 months]. Mean survival was 8.8 months [6-48 months]. Overall survival at 1 year and 2 years was 31% and 16%, respectively (Figure 3).
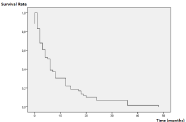
Figure 3: Overall survival according to Kaplan Meier method.
Analytic study
Epidemiological, clinical, biological, morphological and therapeutic parameters were compared between the two groups.
The results of the univariate analysis are resumed in Table 1.
Parameter
Group 1
HCC on viral cirrhosis (N=68)
Group 2 HCC on non viral cirrhosis (N=25)
p
Epidemiological
-Age (mean age in years)
-Sex (Male/Female)
65
43/25
68
15/10
0.599
0.621
Past medical facts
-Diabetes (yes/no)
-Hypertension (yes/no)
-Obesity (yes/no)
18/50
26/42
19/49
5/20
5/20
4/21
0.787
0.210
0.639
Habits
-Smoking (yes/no)
-Alcohol (yes/no)
37/31
18/51
14/11
6/19
0.812
1
Clinical
-Abdominal pain (N=)
-Loss of weigh (N=)
-Screening (N=)
-Decompensation (N=)
-Digestive bleeding (N=)
22
10
33
12
7
10
7
2
6
1
0.319
0.118
<0.001
0.053
0.674
Biological
-ASAT/ALAT (mean)
-GGT (mean)
-PAL (mean)
-Prothrombin time (mean)
-Bilirubin (mean)
-Alphafoetoprotein (mean)
101/55 UI
161 UI
374 UI
62%
66 μmol/L
26494 ng/mL
118/73 UI
97 UI
204 UI
66%
49 μmol/L
20785 ng/mL
0.959
0.142
<0.0001
0.321
0.04
0.984
Endoscopical
-Oesophageal varices (yes/no)
-Red signs (yes/no)
59/9
14/54
20/5
5/20
0.845
1
Morphological
-Number of nodules (mean)
-Size of nodules (mean)
-Portal vein thrombosis (yes/no)
-Metastases (yes/no)
45mm
17/51
6/62
31mm
7/18
3/22
<0.0001
0.589
1
Scores
-Child Pugh (A, B, C)
-OKUDA (1, 2, 3)
-BCLC (A, B, C, D)
-Performance status (0,1-2,3-4)
24/32/12
10/38/20
13/17/19/19
40/25/3
6/11/8
1/9/15
1/9/3/12
11/12/2
0.059
0.018
0.02
0.054
Therapeutic
-Curative treatment (yes/no)
-Palliative treatment (yes/no)
-Symptomatic treatment (yes/no)
-Response au traitement (yes/no)
15
9
44
16/52
2
3
20
4/21
0.053
0.671
0.021
0.438
Survival
-6 months
-12 months
-18 months
43%
20%
7%
62%
43%
30%
0.506
<0.0001
<0.0001
Table 1: Comparison of the two groups (univariate analysis).
Mean survival in groups 1 and 2 was respectively 3.7 and 10.4 months. Log Rank test objectified a significant difference (p=0.005) between the two groups (Figure 4).
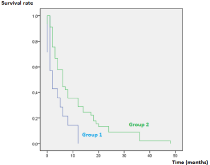
Figure 4: Comparison of survival rates between the two groups.
Discussion
Main etiology of HCC is represented by viral hepatitis B and C especially when occuring on cirrhotic liver [7]. Despite the effectiveness of antiviral treatments, the prevalence of HCC continues to grow. Knowledge of the impact of the etiology of cirrhosis on the prognosis and survival of HCC is essential in order to provide appropriate management, screening and monitoring for each patient.
Our study has shown a significant difference concerning biological parameters, tumor size, prognostic classifications and survival. This indicates a more advanced stage and a poorer prognosis of HCC occuring on viral cirrhosis.
Studies comparing the incidence of HCC in viral cirrhosis compared with non-viral cirrhosis have shown a clear superiority of this incidence in patients with viral hepatitis, especially in American populations compared with Asian populations [8].
Indeed, HCV is the most important risk factor for HCC in Western Europe and North America with an annual incidence of 3 to 8% [9]. The risk of developing HCC on viral hepatitis B is about 10 to 25% [10]. In addition, it has been shown that patients with combined HCV and HBV infection are at a higher risk of developing HCC than those with HCV or HBV alone (2 to 6 times compared to each infection alone) [11] This is explained by additive and multiplicative effect [12]. This co-infection was observed in 3 patients in our cohort.
Literature is more nuanced regarding the epidemiological and clinical characteristics. For example, a Chinese study of 319 patients showed that patients with non-viral cirrhosis HCC tended to be diagnosed at advanced stages but with a lower Child Pugh score than HCC on viral cirrhosis [13]. Another study published in 2003 [14] found similar clinical characteristics in our study since viral cirrhosis HCC were more advanced (with a higher serum alpha-fetoprotein level, a higher prevalence of size tumor higher than 3 cm, multifocal HHC and portal thrombosis).
Histological features may also vary depending on the etiology. Thus, cirrhotic patients can develop two different types of HCC: a nodular type (related to the degeneration of regeneration nodules), independent of etiology, and more infiltrative and aggressive form, related to viral cirrhosis, reflecting direct viral carcinogenesis [15]. This factor was not studied in our cohort since the diagnosis of HCC was based on morphological criteria and liver biopsy was not performed in common practice.
Furthermore, comparing hepatitis B and C, a Korean study published in 2014 showed clear clinical and morphological differences between HBV and HCV-related HCC but no difference in treatment outcome and long-term survival except for very advanced tumors. Another Italian study demonstrated a difference in survival according to the type of virus: the HCC occurring on viral B cirrhosis had a better survival compared to viral C cirrhosis (hazard ratio at 1.5, 95% confidence interval 1-2, 29, p=0.048) [16].
In addition, the etiological management of cirrhosis may also affect carcinogenesis and the prognosis of HCC. Thus, a recent meta-analysis has shown that adjuvant interferon treatment after curative treatment of HCC on viral cirrhosis B improves survival and decreases the rate of recurrence [17]. In addition, the prevalence of HVC has significantly decreased since the development of direct antivirals, but these have been incriminated in the elevation of the risk of HCC with a recurrence rate of nearly 30% according to an Italian study presented by Brilliant and al [18] and confirmed by the study by Brix et al [19]. Thus, HVC-infected cirrhotic patients treated with direct antivirals should be closely monitored. This has not been studied in our series due to the later introduction of direct antivirals in our country.
Therapeutically, the majority of studies have focused on surgical treatment [20-25]. For example, Tanase and al study published in 2014 [21] found no improvement in long-term survival in patients with HCC on viral cirrhosis operated compared to HCC on non-viral cirrhosis. This was also found in Nishikawa and al study in 2013 [22]. However, a meta-analysis published in 2011 including 20 studies [23], found a more reserved prognosis in CHC on viral cirrhosis operated. This difference may be related to a hormonal component since the difference in survival appeared to be greater in women [24] and could also be predicted by the bilirubin level [25]. A significant difference of this biological factor has been found in our study.
No difference was observed in patients who underwent radiofrequency according to their viral status [26].
The risk of recurrence of HCC seems also to differ according to the patient’s viral status, as shown in the study by Koike et al [27]. This study objectified that HCC on viral C cirrhosis recurred more than HCC on viral B or non-viral cirrhosis. In another Japanese study [28], hepatitis C, virus infection was a significant risk factor for intrahepatic recurrence after resection and was also associated with multiple lesions during recurrence. Finally, a large Japanese study of 11950 patients [29] showed a lower rate of recurrent CHC surgery in patients with non-B non-C cirrhosis compared to viral cirrhosis.
The prognosis of patients with HCC was also different according to viral status. Thus, a recent Mexican study published in 2017 [30] didn’t find a difference in overall survival by comparing HCC on viral or non-viral cirrhosis. This was also shown by an Italian study [31]. However, another study showed that overall survival of liver-transplanted HCC with viral B or non-viral cirrhosis was greater than HCC on viral C cirrhosis with a higher rate of hepatic retransplantation [32]. Finally, a Japanese study published in 2010 [33] found a higher mortality of HCC on non-viral cirrhosis. This was due to late detection because of lack of consensus regarding screening in this sub group.
The limits of our study are:
• Its retrospective monocentric nature
• There might be a heterogeneity in the group of viral cirrhosis between viral B and viral C cirrhosis that would be interesting to study by comparing the two subgroups. This was not done because of the limited sample size.
• The impact of treatment of hepatitis (B or C) has not been studied due to the later introduction of direct antiviral agents for hepatitis C in our country.
• It would be interesting to evaluate the carcinogenic cofactors associated with viral infection, especially sex (with hormonal differences) or metabolic syndrome, which could influence the characteristics and prognosis of HCC.
Conclusion
In our study, HHC on viral cirrhosis was more common (73%) and characterized by a higher tumor size, as well as more advanced prognostic scores (OKUDA and BCLC). The survival was lower in the group of viral cirrhosis as evidenced by a higher rate of patients treated symptomatically and poorer survival. It would be interesting to conduct comparative studies of each etiological subtype in order to determine an adapted screening protocol adapted to each etiology. In addition, with the recent development of direct anti-viral drugs, the etiological treatment of cirrhosis should modify the outcome of HCC.
References
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012; 142: 1264-1273.
- Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005; 9: 91-211.
- Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006; 43: 1303-1310.
- Llovet JM, Fuster J, Bruix J; Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004; 10: 115-120.
- Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208-1236.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53: 1020-1022.
- Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF et al. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56: 908-943.
- Mair RD, Valenzuela A, Ha NB, Ayoub WS, Daugherty T, Lutchman GA. Incidence of Hepatocellular Carcinoma among US Patients with Cirrhosis of Viral or Non-Viral Etiologies. Clin Gastroenterol Hepatol. 2012; 10: 1412- 1417.
- Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008; 14: 4300-4308.
- Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP, Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016; 3: 41-53.
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology. 2004; 127: 35-50.
- Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998; 75: 347-354.
- Xiao L, Zhang RL, Zhang H, Tulahong A, Zhang YF, Wen H et al. Comparison of the clinical characteristics and survival between Uyghur patients with hepatitis virus related and non-B, non-C hepatocellular carcinoma in Xinjiang, China. Chin J Cancer Res. 2015; 27: 279-287.
- Dohmen K, Shigematsu H, Irie K, Ishibashi H. Comparison of the clinical characteristics among hepatocellular carcinoma of hepatitis B, hepatitis C and non-B non-C patients. Hepatogastroenterology. 2003; 50: 2022-2027.
- Benvegnu L, Noventa F, Bernardinello E, Pontisso P, Gatta A, Alberti A. Evidence for an association between the aetiology of cirrhosis and pattern of hepatocellular carcinoma development. Gut. 2001; 48: 110-115.
- Cantarini MC, Trevisani F, Morselli-Labate AM, Rapaccini G, Farinati F, Del Poggio P et al; Italian Liver Cancer (ITA.LI.CA) group. Effect of the etiology of viral cirrhosis on the survival of patients with hepatocellular carcinoma. Am J Gastroenterol. 2006; 101: 91-98.
- Xu J, Li J, Chen J, Liu ZJ. Effect of adjuvant interferon therapy on hepatitis b/c virus-related hepatocellular carcinoma after curative therapy - meta-analysis. Adv Clin Exp Med 2015; 24: 331-340.
- Cardoso H, Vale AM, Rodrigues S, Gonçalves R, Albuquerque A, Pereira P et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J Hepatol. 2016; 65: 1070-1071.
- Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016; 65: 719-726.
- Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010; 15: 14-22.
- Tanase AM, Dumitrascu T, Dima S, Grigorie R, Marchio A, Pineau P et al. Influence of hepatitis viruses on clinicopathological profiles and longterm outcome in patients undergoing surgery for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2014; 13: 162-172.
- Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Comparison of clinical characteristics and survival after surgery in patients with non B and non C hepatocellular carcinoma and hepatitis virus-related hepatocellular carcinoma. J Cancer. 2013; 4: 502-513.
- Zhou Y, Si X, Wu L, Su X, Li B, Zhang Z. Influence of viral hepatitis status on prognosis in patients undergoing hepatic resection for hepatocellular carcinoma : a meta-analysis of observational studies. World J Surg Oncol. 2011; 9: 108.
- Li T, Qin LX, Gong X, Zhou J, Sun HC, Qiu SJ et al. Hepatitis B virus surface antigen-negative and hepatitis C virus antibody-negative hepatocellular carcinoma: clinical characteristics, outcome, and risk factors for early and late intrahepatic recurrence after resection. Cancer. 2013; 119: 126-135.
- Hiwatashi K, Ueno S, Sakoda M, Iino S, Minami K, Yamasaki Y et al. Problems of Long Survival Following Surgery in Patients with Non B Non C HCC: Comparison with HBV and HCV Related-HCC. J Cancer. 2015; 6: 438-447.
- Chen PH, Kao WY, Chiou YY, Hung HH, Su CW, Chou Y et al. Comparison of prognosis by viral etiology in patients with hepatocellular carcinoma after radiofrequency ablation. Ann Hepatol. 2013; 12: 263-273.
- Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M et al. Risk Factors for Recurring Hepatocellular Carcinoma Differ According to Infected Hepatitis Virus-An Analysis of 236 Consecutive Patients With a Single Lesion. Hepatology. 2000; 32: 1216-1223.
- Wakai T, Shirai Y, Yokoyama N, Nagakura S, Hatakeyama K. Hepatitis viral status affects the pattern of intrahepatic recurrence after resection for hepatocellular carcinoma. Eur J Surg Oncol. 2003; 29: 266-271.
- Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N et al. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg. 2015; 261: 513-520.
- Alkhalili E, Greenbaum A, Luo L, Rodriguez R, Caldwell K, Estrada OM et al. Viral hepatitis status does not affect survival in patients with hepatocellular carcinoma. Ann Gastroenterol. 2017; 30: 101-105.
- Trevisani F, Magini G, Santi V, Morselli-Labate AM, Cantarini MC, Di Nolfo MA et al; Italian Liver Cancer (ITA. LI.CA) Group. Impact of etiology of cirrhosis on the survival of patients diagnosed with hepatocellular carcinoma during surveillance. Am J Gastroenterol. 2007; 102:1022-1031.
- Groeschl RT, Hong JC, Christians KK, Turaga KK, Tsai S, Pilgrim C et al. Viral status at the time of liver transplantation for hepatocellular carcinoma: a modern predictor of long term survival. HPB (Oxford). 2013; 15: 794-802.
- Akahoshi H, Taura N, Ichikawa T, Miyaaki H, Akiyama M, Miuma S et al. Differences in prognostic factors according to viral status in patients with hepatocellular carcinoma. Oncol Rep. 2010; 23: 1317-1323.
