Abstract
We present a 44-year-old female with an adrenal adenoma characterized by late autonomous secretion of aldosterone. The patient was admitted at our Hypertension Unit for a resistant form of severe hypertension with target organ damage and a history of adrenal non-functioning adenoma. Seven years before admission, the patient was evaluated for a mild normokalemic hypertension associated with accessional headache and a diagnosis of essential hypertension and left adrenal incidentaloma was made. After few years of well-controlled hypertension, blood pressure levels worsened and a significant cardiac remodeling and a second grade retinopathy appeared despite of the increased number of antihypertensive drugs. Hormone tests were then repeated and showed an elevated aldosterone to renin ratio with normal cortisol and catecholamines. Primary aldosteronism was confirmed by the lack of suppression of aldosterone levels after an intravenous saline loading test. Computerized tomography scanning confirmed the left adrenal adenoma that was increased respect to the previous evaluation. Successful adrenalectomy was performed, which resulted in a decrease of blood pressure and no need of antihypertensive drugs. This case-report confirms the need for an accurate diagnostic work-up for primary aldosteronism and a strict follow-up of patients with mild hypertension and apparently non-functioning adrenal adenoma.
Introduction
Adrenal adenomas are occasionally found in patients with hypertension, a condition that rises the diagnostic problem to rule out a primary aldosteronism or other endocrine forms of secondary hypertension. Once exclusion of a functioning adenoma has been made by an extensive diagnostic work-up, there is no evidence on the correct timing for the follow-up of these patients. This case report confirms that patients with an apparently non-functioning adrenal adenoma can be at risk for developing resistant hypertension and target organ damage with the adenoma becoming functioning over time.
Case Presentation
A 44-year-old female was admitted to our Hypertension Unit for the evaluation of a resistant form of hypertension. The patient had been found to be hypertensive seven years before the present admission when she experienced a progressively worsening of a chronic headache associated with a mild hypertension. In that occasion, she did not report other associated symptoms including flushing, diaphoresis and palpitations, there was no family history of endocrine neoplasms or early cerebral and cardiovascular disease, but her mother was hypertensive too. She was a smoker of about 20 cigarettes/day but she had no other cardiovascular risk factors and a pregnancy occurred few years before was uncomplicated. After an adequate wash-out from anti-hypertensive drugs, urinary catecholamines, plasma Active Renin (AR), potassium and aldosterone levels, 24h urinary free cortisol, midnight serum cortisol and 1-mg overnight dexamethasone suppression test were all within the normal range. Abdominal Computerized Tomography (CT) scanning performed with the suspect of a pheochromocytoma detected the presence of a 16 mm mass inside the left adrenal gland. In that period, our laboratory did not perform metanephrines evaluation and the pheochromocytoma was further excluded by a negative metoclopramide stimulation test [1]. The assessment of hypertensive damage of heart, arteries, kidney and retina was negative. The patient was discharged in satisfactory blood pressure control with only amlodipine 5 mg/day and a diagnosis of essential hypertension and left adrenal incidentaloma was made. The patient did not continue the follow-up at our Hypertension Unit. (Table 1) summarizes laboratory tests and (Table 2) echocardiographic results. (Figure 1A) shows the CT image of the 16 mm left adrenal gland detected during the first evaluation.
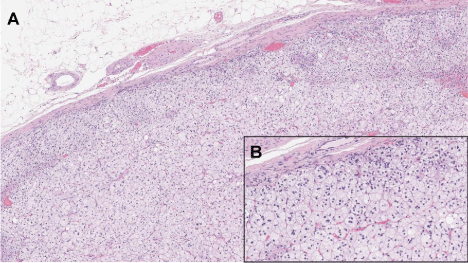
Figure 1: Abdominal computerized tomography scanning that shows left
adrenal adenomas (star) of 16-mm during the first (A) and of 20-mm during
the second (B) assessment seven years later.
Variable
I evaluation
II evaluation
Normal range*
Age (years)
37
44
-
Body weight (Kg)
90
99
-
Body mass index (Kg/m2)
28.4
31.2
-
Smoke (cigarettes/day)
20
20
-
Blood pressure (mm Hg)
150/100
180/110
<140/90
Plasma glucose (mg/dl)
81
79
76-110
Total cholesterol (mg/dl)
127
143
120-240
LDL-Cholesterol (mg/dl)
57
76
<130
HDL-Cholesterol (mg/dl)
61
54
45-65
Triglycerides (mg/dl)
45
65
40-150
Plasma creatinine (mg/dl)
0.76
0.80
0.4-1.3
eGFR CKD-EPI (ml/min/1.73m2)
100
90
>90
Plasma potassium (mmol/l)
3.8
4.1
3.5-5.1
Urinary sodium (mmol/day)
222
81
40-220
Urinary potassium (mmol/day)
44
22
25-125
Urinary sodium to potassium ratio
5
3.7
>3
Urinary protein (mg/day)
78
28
0-300
Microalbuminuria (mg/day)
13
6.5
<30
Plasma active renin (mU/l)
9.5
1.4
2.8-39.9
Plasma aldosterone (ng/dl)
22.4
29.5
1.0-15.0
Plasma aldosterone to renin ratio (ng/dl/mU/l)
2.4
21.1
<3.7
Urinary aldosterone (µg/day)
11
1.5
2.8-30
Plasma aldosterone after saline loading test (ng/dl)
-
28.4
<5
Plasma cortisol after dexamethasone test (nmol/l)
22
20
<50
Urinary epinephrine (nmol/day)
46
16
0-100
Urinary norepinephrine (nmol/day)
446
97
71-508
Urinary dopamine (nmol/day)
3245
1979
400-3700
*Normal range refers to the second evaluation since the clinical laboratory had changed references and unit of measurement over time. eGFR: Estimated Glomerular Filtration Rate With CKD-EPI Formula; LDL: Low Density Lipoproteins; HDL: High Density Lipoproteins
Table 1: Clinical and biochemical characteristics with laboratory normal range during the first and second evaluation of our patient.
Variable
I
evaluation
II
evaluation
LV end-diastolic diameter (mm)
52
54
LV end-systolic diameter (mm)
35
35
Interventricular septum (mm)
9.0
8.7
Posterior LV wall (mm)
7.0
11.1
LV mass (g)
145
204
LV mass indexed* (g/m2)
70
94
Relative wall thickness
0.27
0.41
LV ejection fraction (%)
60
64
E-wave to A-wave velocity ratio (E/A)
1.00
1.08
E-wave deceleration time (ms)
147
270
Left atrium volume indexed* (ml/m2)
12
25
Aortic root (mm)
30
30
*Indexed for the body surface area. LV: Left Ventricle
Table 2: Echocardiographic variables during the first and second evaluation of our patient.
After few years of well-controlled hypertension, the patient developed a progressive worsening of blood pressure despite of the increased number of antihypertensive drugs. She referred again to our Hypertensive Unit and her home blood pressure diary showed mean values of 160/100 mm Hg while she was taking lercanidipine 10 mg/day, olmesartan 20 mg/day, hydrochlorothiazide 12.5 mg/day, doxazosine 4 mg/day and carvedilol 50 mg/day. We decided to repeat hormonal tests after a wash-out period of three weeks with only doxazosine 8 mg/day and verapamil 240 mg/day. On examination, her blood pressure was found to be not controlled (180/110 mmHg) and her body mass index was increased in seven years from 28 to 31 Kg/m2. The rest of the examination was unremarkable. Routine biochemical data were all within normal range. In particular, normal values were seen in plasma levels of sodium, potassium and creatinine. (Table 1) summarizes laboratory data for this second evaluation. Urinary cortisol and catecholamines, thyroid function, adrenocorticotropin, urinary catecholamines and serum cortisol showed normal values. A low dose dexamethasone test showed a normal suppression of cortisol secretion. AR of 1.4 mU/l was below the lower limit of detection and Plasma Aldosterone Concentrations (PAC) 29.5 ng/dl was high, with an Aldosterone to Renin Ratio (ARR) suggestive of autonomous aldosterone secretion. A primary aldosteronism was confirmed by an intravenous saline loading test where infusion of two liters of normal saline within four hours did not suppressed PAC (Table 1). Echocardiographic evaluation showed a significant increment of left ventricular mass and an initial left diastolic dysfunction (Table 2). A grade 2 (Keith-Wagener-Barker) hypertensive retinopathy was detected by funduscopic examination. CT scanning of the adrenal glands confirmed a 20 mm left adrenal mass (Figure 1B) and the diagnosis of aldosterone-producing adrenal adenoma was made. The patient underwent left laparoscopic adrenalectomy and histopathological analysis demonstrated a single well-defined encapsulated yellow nodule showing the typical features of an adrenocortical adenoma (Figure 2). Following surgery, patient recovered well with no need for anti-hypertensive treatment and a monthly clinical and biochemical follow-up had been instituted for the first six months.
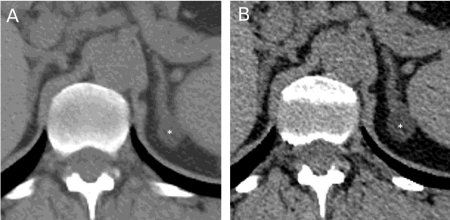
Figure 2: Hematoxylin-eosin stained left adrenal cortical adenoma at 25X (A)
and 250X (B) Magnification.
Discussion
We have presented a case of normokalemic well-controlled hypertensive patient with an apparently non-functioning adrenal adenoma that became functioning during a long-term follow-up period and produced a resistant form of hypertension complicated with target organ damage. Adrenal “Incidentaloma” (AI) is an asymptomatic adrenal mass occasionally discovered by imaging performed for other reasons than assessing adrenal glands. The prevalence of AI depends on age: from less than 1% in subjects younger than 40 years up to 15% in those older than 70 [2]. However, this prevalence can increase to 25% in the presence of hypertension and it has been demonstrated that 66% of patients with AI show a hypertensive disease [3,4]. Aldosterone-producing adenoma is the most frequent form of secondary hypertension occurring in up to 10% of hypertensive patients [5], 36% of hypertensive patients with AI and in more than 50% of patients with AI that were studied for a suspected secondary hypertension [6]. These data suggest that the probability to find a primary aldosteronism in a young hypertensive patient with AI is pretty high and an accurate work-up examination in this context should be always performed [7].
During the first assessment of our patient, accordingly with guidelines pheochromocytoma was excluded by 24h urinary catecholamines levels and a metoclopramide test, cortisol overproduction by 1 mg overnight dexamethasone suppression test and aldosterone overproduction by the finding of normal potassium levels and a normal ARR. The natural history of this patient may suggest that the adrenal adenoma became functioning over time, though this probability derived from the literature is very low (less than 1%) [8]. Another more intriguing hypothesis is that an initial form of aldosterone-producing adenoma was already present from the first evaluation but it was undiagnosed. This second hypothesis is also suggested by the success of adrenalectomy that completely cured hypertension. In this context, an important point is how we might have correctly interpreted ARR cutoff values for the diagnosis of primary aldosteronism in such as a high risk patient. An ARR of 2.4 ng/dl/mU/l was considered normal because lower than the common limit of 3.7 ng/dl/mU/l (converted from 30 ng/dl/ng/ml/h) and 24h urinary aldosterone corrected for sodium excretion was not strongly suggestive for a primary aldosteronism according to the Mayo Clinic criteria [9]. Additionally, no other known causes of false negative ARR were present such as the intake of a low sodium diet and the use of dihydropyridinc calcium channel blockers [10,11]. However, measurement of 24h urinary sodium and aldosterone levels is not always accurate because it depends on an adequate urinary collection and other groups have suggested for the positive screening of primary aldosteronism the lower ARR cutoff value of 2.4 ng/dl/mU/l [5]. With hindsight, in this case the high suspect for a primary aldosteronism should have brought us to perform a saline loading test even if not strictly indicated by the guidelines.
During seven years without a follow-up our patient worsening her blood pressure control and developed significant cardiac remodeling and retinopathy despite of increasing anti-hypertensive treatment. Also, her PAC, ARR and radiological dimensions of adenoma significantly increased over time. This scenario highlights the possibility that a mild form of primary aldosteronism induced by the aldosterone-producing adenoma can be progressively worsened over time by changing cardiovascular hemodynamics and stimulating cardiac and retinal arteries remodeling. What had influenced this progression is not clear and no studies have addressed this point. Our patient increased significantly her body weight in seven years and she continued to smoke about one packet a day; this could have directly influenced aldosterone overproduction and blood pressure increment and contributed to the target organ damage [12]. On the other hand, hyperaldosteronism is strictly associated with metabolic syndrome so that aldosterone overproduction could have directly induced the increment of both body weight and blood pressure over time [13]. Aldosterone excess in primary aldosteronism is also cause of organ damaged inappropriately worse than that expected for the duration of hypertension and for blood pressure levels [14]. Most importantly, both metabolic abnormalities and organ damages improve after adrenalectomy or the use of anti-mineralocorticoids agents [15]. The significant cardiac remodeling in our patient is most probably related to the longer exposure to aldosterone excess and having started a mineralocorticoid antagonist or performed adrenalectomy seven year before could have resolved hypertension and limited cardiac damages. However, starting a causal treatment for a mild form of primary aldosteronism in a patient with well-controlled hypertension and without organ damage is still a matter of debate [16].
More than 40% of aldosterone-producing adenomas are caused by a somatic mutation in a single gene (KCNJ5) and more causal somatic mutations on other genes have been later identified [17]. We could speculate that new additional somatic mutations have been occurred over time contributing to the progression of the adrenal dysfunction and consecutively its growth [18]. Rarely, an adrenocortical carcinoma can appear as a normal functioning adenoma with primary aldosteronism that after adrenalectomy can relapse with a metastatic presentation [19]. In our patient, the left adrenal mass increases of about 25% in seven years which for the current guidelines on the follow-up of AI is a criterion for suspecting malignancy and performing surgical excision [20]. However, the slow time of mass enlargement and the absence of malignant aspects during histological analysis seemed to rule out a carcinomatous degeneration. Unfortunately, we did not perform a genetic characterization of the excised adenoma to assess further this point.
Conclusions
This case raises some points that in conclusion should be highlighted. First, the use of a fixed cutoff for ARR without taking into account clinical history and characteristics of the patient should be applied with caution. In our case, despite of a normal ARR, the low prevalence of AI in the young, the high prevalence of primary aldosteronism in hypertensive patients with AI and the relative high baseline PAC should have suggested a second level confirmation test for excluding the disease. Second, provided that a primary aldosteronism had been diagnosed, it is not clear whether it is worth to consider a treatment for mild forms of the disease and possibly which type of treatment (surgery versus mineralocorticoid receptor antagonists) would be better. Future studies addressing these points are needed.
References
- Hsu TS, Lee CP, Kuo CT. Diagnostic use of metoclopramide in hypertension caused by pheochromocytoma. Int J Cardiol. 1993; 42: 79-86.
- Arnaldi G, Boscaro M. Adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012; 26: 405-419.
- Gouli A, Kaltsas G, Tzonou A, Markou A, Androulakis II, Ragkou D, et al. High prevalence of autonomous aldosterone secretion among patients with essential hypertension. Eur J Clin Invest. 2011; 41: 1227-1236.
- Hedeland H, Ostberg G, Hokfelt B. On the prevalence of adrenocortical adenomas in an autopsy material in relation to hypertension and diabetes. Acta Med Scand. 1968; 184: 211-214.
- Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016; 101: 1889-1916.
- Pappa T, Papanastasiou L, Kaltsas G, Markou A, Tsounas P, Androulakis I, et al. Pattern of Adrenal Hormonal Secretion in Patients with Adrenal Adenomas: The Relevance of Aldosterone in Arterial Hypertension. J Clin Endocrinol Metab. 2012; 97: 537-545.
- Monticone S, Viola A, Tizzani D, Crudo V, Burrello J, Galmozzi M, et al. Primary aldosteronism: who should be screened? Horm Metab Res. 2012; 44: 163-169.
- Cawood TJ, Hunt PJ, O’Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol. 2009; 161: 513-527.
- Young WF, Hogan MJ, Klee GG, Grant CS, van Heerden JA. Primary aldosteronism: diagnosis and treatment. Mayo Clin Proc. 1990; 65: 96-110.
- Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary Sodium Restriction Increases the Risk of Misinterpreting Mild Cases of Primary Aldosteronism. J Clin Endocrinol Metab. 2016; 101: 3989-3996.
- Mulatero P, Rabbia F, Milan A, Paglieri C, Morello F, Chiandussi L, et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. 2002; 40: 897-902.
- Dinh Cat AN, Friederich-Persson M, White A, Touyz RM. Adipocytes, aldosterone and obesity-related hypertension. J Mol Endocrinol. 2016; 57: 7-21.
- Fallo F, Pilon C, Urbanet R. Primary aldosteronism and metabolic syndrome. Horm Metab Res. 2012; 44: 208-214.
- Catena C, Colussi G, Brosolo G, Novello M, Sechi LA. Aldosterone and Left Ventricular Remodeling. Horm Metab Res. 2015; 47: 981-986.
- Catena C, Colussi G, Sechi LA. Treatment of Primary Aldosteronism and Organ Protection. Int J Endocrinol. 2015; 2015: 597247.
- Ito Y, Takeda R, Takeda Y. Subclinical primary aldosteronism. Best Pract Res Clin Endocrinol Metab. 2012; 26: 485-495.
- Zennaro M-C, Boulkroun S, Fernandes-Rosa F. An update on novel mechanisms of primary aldosteronism. J Endocrinol. 2015; 224: 63-77.
- Nanba K, Chen AX, Omata K, Vinco M, Giordano TJ, Else T, et al. Molecular Heterogeneity in Aldosterone-Producing Adenomas. J Clin Endocrinol Metab. 2016; 101: 999-1007.
- Hussain S, Panteliou E, Berney DM, Carpenter R, Matson M, Sahdev A, et al. Pure aldosterone-secreting adrenocortical carcinoma in a patient with refractory primary hyperaldosteronism. Endocrinol Diabetes Metab Case Rep. 2015; 2015: 150064.
- Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016; 175: 1-34.
