Abstract
The term of Sudden Cardiac Death (SCD) is a special situation occurred when a person dies suddenly and unexpectedly from cardiovascular diseases. Hypertrophic Cardiomyopathy (HCM), coronary artery abnormalities, myocarditis, arrhythmogenic right ventricular dysplasia, ion channel defects and aortic stenosis can all cause etiopathogenesis of SCD. The most common reason of sudden death in athletes is Hypertrophic Cardiomyopathy (HCM) which caused by mutations in genes encoding sarcomeric proteins. Here, we review the genetic basis of sudden cardiac death with a focus on the current knowledge on the genetics of the HCM by mutations in genes.
Introduction
Sudden cardiac death
Sudden Cardiac Death (SCD) is natural and unexpected death from cardiac causes, heralded by abrupt loss of consciousness within 1hour of the onset of an acute change in cardiovascular status [1]. It is difficult to estimate the incidence of SCD but cardiovascular diseases are responsible for approximately 17 million deaths every year in the world, approximately 25% of which are SCD [2]. Several factors like age, race, gender and heredity influence the incidence of SCD. The risk of SCD is higher in men than in women and it increases with age due to the higher prevalence of Coronary Artery Disease (CAD) in older age. Cardiac diseases associated with SCD differ in young vs. older individuals. In the young, there is a predominance of channelopathies and cardiomyopathies, myocarditis and substance abuse, while in older populations, chronic degenerative diseases predominate (CAD, valvular heart diseases and heart failure) [3].
Causes of SCD are numerous: atherosclerotic coronary artery disease, nonatherosclerotic coronary abnormalities, ventricular hypertrophy of myocardium including hypertrophic cardiomyopathy, myocardial diseases and heart failure, congenital heart diseases and diseases of cardiac valves, electrophysiological abnormalities of cardiac conduction system (eg: Wolff Parkinson White Syndrome, Brugada Syndrome, long QT interval syndromes) and miscellaneous causes including extreme physical activity, blunt chest trauma(commotio cordis) and aortic dissection [1].
Sudden cardiac death in athletes
Athletes appear at excessive risk of SCD compared with similar aged non-athletes [4]. The annual incidence of SCD in young athletes (<35 years) is estimated to range from 0.7 to 3.0 per 100000 athletes [5]. In older athletes the incidence is higher and is expected to increase with age [6]. The most frequent causes of sudden death in younger athletes are inherited arrhythmogenic disorders (cardiomyopathies and channelopathies) and CAD (both congenital and acquired). In older athletes (age 35-40 years), as in the general population, coronary atherosclerotic disease accounts for most of the cases [7].
Careful history taking to uncover underlying cardiovascular disease, rhythm disorders, syncopal episodes or family history of SCD is recommended in athletes and upon identification of ECG abnormalities suggestive of structural heart disease, echocardiography and/or CMR imaging is recommended as class I recommendation [3].
Hypertrophic Cardiomyopathy (HCM)
HCM is the most common genetic cardiovascular disease, caused by a multitude of mutations in genes encoding proteins of cardiac sarcomere. HCM is most frequently transmitted as an autosomal dominant genetic trait, most studies report a small male preponderance and the frequency of HCM in different racial groups is similar [8].
HCM is characterized by increased Left Ventricular (LV) wall thickness without ventricular dilatation that is not solely explained by abnormal LV loading conditions. LV hypertrophy has diverse patterns of asymmetria and develops dynamically after a variable period of latency. Clinical course is also variable; it may remain stable for long periods but may cause sudden, unexpected death, progressive heart failure or arrhythmias [8].
HCM is found to be the most common cause of SCD in young competitive athletes in Unites States, being responsible for one thirds of events [9]. Risk stratification has an important role in patient management and prevention of SCD. Presence of family history of sudden death, extreme LV hypertrophy, unexplained syncope, nonsustained ventricular tachycardia and abnormal blood pressure response are the risk factors for SCD [10]. Patients with HCM should be advised against participation in sports and discouraged from intense physical activity, especially when they have recognized risk factors for SCD or a LV outflow gradient Avoidance of competitive sports is recommended in patients with HCM in order to prevent SCD in ESC 2015 guideline [3].
Similarly 2015 ACC/AHA guideline recommends that athletes with clinical expression and diagnosis of HCM should not participate in most competitive sports independent of age, sex, magnitude of LV hypertrophy and outflow tract obstruction. It also advises against use of medical treatment or implantable cardioverter-defibrillator to control cardiac symptoms for purpose of permitting participation in competitive sports. Different from previous recommendations, 2015 ACC/AHA guideline recognizes atheletes with genotypepositive, phenotype-negative HCM (carrying the gene but having no clinical manifestations of the disease) without a family history of SCD and states that they may participate in athletics with class II (a) recommendation [11].
Athlete’s Heart
It is revealed that there is a correlation between high level of physical training with morphological and functional cardiac alterations which is called athlete’s heart [12,13]. Cardiac enlargement in both left and right side of athletes’ heart has been detected first by basic physical examination with percussion of the chest by the end of 19th century; then confirmed with radiography and necroscopy [14]. These findings were enlarged with electrocardiogram and other advance techniques such as magnetic resonance imaging [15].
Cardiovascular features of heart can change depending on the style of sports. Endurance exercises (also known as dynamic, isotonic, or aerobic) such as long-distance running or swimming, cause decreased peripheral vascular resistance depending on maximum oxygen consumption, cardiac output, stroke volume and systolic blood pressure [16,17]. If these exercises are performed for long-term periods, adaptations such as cardiac output and arteriovenous oxygen difference which cause increased maximal oxygen uptake; will occur. Therefore, the volume load on the Left Ventricle (LV) is observed in endurance performance athletes’ heart. On the other hand, the exercises such as wrestling, weightlifting, or throwing heavy objects are in the group of strength training (also known as static, isometric, power, or anaerobic) [16,17]. In this condition; while oxygen consumption and cardiac output increase steadily, blood pressure, peripheral vascular resistance and heart rate increase significantly. The little or no increase in oxygen uptake is observed in long-term exercised athletes and it causes generally a pressure load [16]. As in LV, Right Ventricule (RV) is also expected to accept and eject larger volume of blood. Extended RV cavities and RV wall thickness are observed in endurance athletes [18-20]. The studies couldn’t show important differences between strength-trained athletes and controls in terms of RV morphology. These two groups can be distinguished from each other with RV inflow tract diameter, RV end diastolic area and tricuspid inflow velocity deceleration time [20,21]. It is generally showed that there are also aortic root alterations in athletes. Endurance athletes have high-volume aortic flow with modest systolic blood pressure elevation. Besides, strength-trained athletes have normal volume aortic flow with profound elevation of systolic blood pressure [20]. In combination athletes; the features of endurance and strength athletes are overlapping [22].
Individual morphology and physiology factors are also so important in cardiac alterations. To determine cardiac risk profile of athlete, some individual factors such as medical history, personal family history, type and intensity of athletic activity, supine and standing blood pressure, heart rate, venous and arterial examination and cardiac auscultation; should be recorded because these factors affect the heart conditions [20]. Another factor which affects the feature of heart can be race because studies have shown individual differences in wall thickness between white and black athletes [20].
Genetics of Sudden Cardiac Death
During the 20 years, conducted genetic studies have provided insight into understanding the inherited cardiac disorders associated with sudden cardiac death. Although the major cause of SCD after the age of 45 years is coronary artery disease, the basis of the SCD usually are rare hereditary cardiac disorders in the pediatric population and in young adults. These disorders are divided into two main classes, namely the cardiomyopathies and the primary electric disorders (Table 1) [23,24].
Phenotype
Related genes
Mode of inheritance
Cardiomyopathies
Hypertrophic Cardiomyopathy
MYH7, MYBPC3, TNNTT2, TNNI3, TTN, ACTC1
autosomal dominant
Dilated Cardiomyopathy
LMNA, TNNT2, TTN, DES, MYH7, PLN, TCAP
autosomal dominant
autosomal recessive
X-linked
Arrhythmogenic Cardiomyopathy
DSP, PKP2, DSG2, DSC2, JUP
autosomal dominant
Primary Electric Disorders
Long-QT Syndrome
KCNQ1, KCNH2 , SCN5A, ANK2, KCNE1, KCNE2, CAV3, SCN4B, AKAP9, KCNJ5
autosomal dominant
Short-QT Syndrome
KCNQ1, KCNH2, KCNJ2, CACNA1C, CACNB2, CACNA2D1
autosomal dominant
Brugada Syndrome
SCN5A, GPD1L, SCN1B,SCN3B, MOG1, KCND3, KCNE3, KCNE5 , CACNA1C, CACNB2,KCNJ8
autosomal dominant
Catecholaminergic Polymorphicventricular tachycardia
RYR2, CASQ2, TRDN
autosomal dominant
autosomal recessive
Table 1: Genes that cause cardiomyopathies and primary electric disorders.
Cardiomyopathies and the primary electric disorders have been classically considered Mendelian disorders wherein a potent monogenic component contributes substantially to risk. Without exception the various cardiomyopathies and primary electric disorders are genetically heterogeneous. For some of the rare cardiac disorders, the notion that they are Mendelian is now being questioned. For these disorders, a somewhat more complex genetic inheritance (oligogenic model) is now suspected. In contrast to monogenic paradigma, the genetic susceptibility for these disorders may be determined by the cumulative effect of multiple genetic variants (Figure 1) [23].
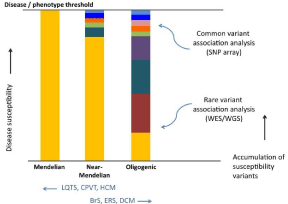
Figure 1: The likely continuum of complexity of genetic architecture in the
rare inherited cardiac disorders.
Genetics has played a very important role in the understanding of inherited predisposition to SCD, since the Landmark discoveries of the first genes for Hypertrophic Cardiomyopathy (HCM) by Seidman at alin the early to mid-1990s [25]. Hypertrophic Cardiomyopathy (HCM) is a familial disease that in fifty percent of the cases is inherited in an autosomal dominant pattern. Mutations in any of the several sarcomeric genes lead to HCM. [26,27]. The disorder has been predominantly linked to mutations in genes encoding components of the sarcomere. A sarcomere gene mutation is identified in 50% to 60% of cases, with the MYBPC3 or MYH7 gene being most commonly involved [28]. Albeit with varying evidence for causality, several genes encoding nonsarcomeric proteins have also been reported in patients with HCM, including Z-disk proteins (eg, ACTN2 and MYOZ2) and intracellular calcium modulators (eg, JPH2) [29].
Dilated Cardiomyopathy (DCM) is the most common cause of congestive heart failure in young patients. The pattern of inheritance is variable, so the patients present both locus heterogeneity and allellic heterogeneity. Mutations in many genes have been reported to cause different forms of dilated cardiomyopathy. Therefore, autosomal dominant, autosomal recessive and X-linked inheritance can be observed. However, the autosomal dominant pattern is the most frequent mode of inheritance [26]. DCM is genetically heterogeneous with >30 disease genes being reported to date. These genes encode a wide range of proteins, with the following 4 genes accounting for the majority of genotype positive cases: Titin (TTN), Lamin A/C (LMNA), β-Myosin Heavy Chain (MYH7) and Cardiac Troponin T (TNNT2) [30]. When the mutation is in one of the sarcomeric genes (MYH7) the affected patients are usually young adults [31].
Arrhythmogenic Cardiomyopathy (ACM) is an autosomal dominant disorder and it has been estimated that ≈30% to 50% of patients harbor a putative mutation in 1 of 5 genes encoding desmosomal proteins: Plakophillin (PKP2), Desmoplakin (DSP), Plakoglobin (JUP), Desmoglein-2 (DSG2) and Desmocollin-2 (DSC2), with the most commonly involved being PKP2 [32].
The LQTS, which frequently presents in childhood is most commonly inherited in an autosomal dominant fashion, genetically heterogeneous and can be caused by mutations in several genes encoding voltage-gated K+ channel subunits (KCNQ1, KCNH2, KCNE1, KCNE2) [33], voltage-gated Na+ channel subunits (SCN5A, SCN4B) [34], an L-type Ca2+ channel (CACNA1C) [35], inwardly rectifying K+ channels (KCNJ2, KCNJ5) [36] and various channelinteracting proteins (ANK2, CAV3, AKAP9, SNTA1) [37]. LQT1 is the most common form of LQTS, accounting for ≈35% of cases. It arises from loss-of-function mutations in the KCNQ1 gene, which encodes the slowly activating delayed rectifier current (IKs). LQT2, accounting for 30% of cases, arises from loss-of-function mutations in KNCH2 (also known as HERG), encoding the rapidly activating delayed rectifier current (IKr). Gain-of-function mutations in SCN5A that lead to an increase of the late sodium current (INa) underlie LQT3 and are found in ≈10% of probands [23]. Between them, these 3 genes account for ≈90% of genotype-positive LQTS patients [38].
Short-QT syndrome is a familial disease that is characterized by a high incidence of sudden death. Mutations in six different genes encoding either K+ channel (KCNQ1, KCNH2, KCNJ2) [39,40] or Ca2+ channel (CACNA1C, CACNB2, CACNA2D1) [41,42] subunits have been associated with this phenotype.
The Brugada Syndrome (BrS), which is inherited in an autosomal dominant pattern, is associated with sudden death in young people as the patients have malignant ventricular tachyarrhythmias and sudden cardiac death. Mutations in SCN5A encoding the α-subunit of the cardiac voltage-gated sodium channel were the first-identified genetic cause of BrS [26]. A recent comprehensive mutational analysis of 12 known BrS-susceptibility genes in a large cohort of unrelated BrS patients identified SCN5A mutations in 16%, with the other 11 genes accounting for <5% of patients [43]. The majority (≈80%) of BrS, therefore, still remains genetically unresolved.
Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) is an inherited tachyarrhythmia that is caused by acute adrenergic activation during exercise or acute emotion in young adolescents. It presents locus heterogeneity and in only approximately 50% of the cases the mutations in the genes causing the disease has been identified. In the presence of a clear diagnosis, a RYR2 mutation, with autosomal dominant inheritance, is found in ≈60% of patients with CPVT [26].
The identification of genetic factors that predispose to SCD is important because this enables genetic testing that may contribute to diagnosis and risk stratification. The identification of genetic risk factors also provides molecular leads that trigger an increased understanding of disease pathways underlying SCD and development of new therapies.
The Relation between Sports and Sudden Cardiac Death
Physical exercise is one of the most healthful tools that have positive effects on blood lipids, blood pressure, insulin resistance and overweight. It has been proven with epidemiologic studies that moderate aerobic exercises lowers the chances of coronary artery diseases. Although there has been some controversial relations suggested between intense endurance exercises and some cardiac diseases (e.g. atrial fibrillation, ventricular fibrosis), for majority of the population, exercise tends to be an activity that positively affects health [44]. Sometimes co occurrence can be seen between sudden cardiac death and sport activities which, by certain regulations, aim success and perfection and consist of varying physical and mental challenges according to different branches.
Since ancient times, athletes were considered as heroes in both small communities and international arenas. So much so that in Ancient Greece, not only they were admired by all, sometimes they were even believed to be gods racing amongst mortals. For this reason, sudden death among athletes who are praised and believed to be strong becomes paradoxical and magnifies the effect of the situation [45].
SCD is the single most upsetting thing that can happen during sport activities. In addition to being a devastating tragedy for his/her family, teammates, sports club and fans, considering current media coverage, it is also a disheartening factor for people who are starting or continuing to pursue their sports career [46].
There is no single agreement on the definition of sudden death. According to its forensics definition, all deaths that are seen by witnesses and occur in a range from couple of minutes to couple of hours are considered “sudden death”. World Health Organization (WHO), defines sudden death by exercise as deaths that occur in a range from thirty seconds to six hours after an exercise [47]. Another argument would suggest that sudden death is any death that occurs unexpectedly, without any traumatic event, to a healthy person in an hour after the symptoms start [46,48]. The first recorded sudden death after a physical activity in history is Pheidippides, a courier in the Greece army who, after the Battle of Marathon in 490 BC, has ran all the way to Athens to deliver the message of triumph, only to fall down to his death after the delivery. To honor Pheidippides, scanning programs for the athletes are named after him [49,50].
Sudden death in athletes shows itself as an increasing health problem as population of athletes has risen all over the world. There is a consensus of thought regarding sudden cardiac death being the leading cause for deaths of athletes on the field [51]. Nevertheless, there is varying data regarding frequencies of sudden death related to cardiovascular diseases in sports across the ages. While the studies among athletes in their high school ages show varying numbers between 1/100.000 and 1/300.000, another study in Italy from 1976- 96 among athletes under the age of 35 shows the frequency of death incidence as 0.8/100.000. This numbers reach up to as high as 1/50.000 as the athletes age and cardiovascular risk factors which affect sudden death frequency increase [52-55].
When athletes and people who don’t engage in sports activities were compared, sudden death frequency in athletes were up to two and a half times bigger than the others; and this further suggests that sports facilitates sudden death in these individuals. Sudden death occurs more commonly in basketball, soccer and football players and 8-9 times more in male athletes than females [17,49,56].
Sports activities are usually classified by the intensity (low, medium, high) of their dynamic (isotonic) or static (isometric) exercise which is required to perform it. Dynamic exercises are exercises that big muscle groups produce relatively smaller power, cause changes to the length of the muscles and joint movements, while don’t make any changes to the tension of the muscles (e.g. running). Whereas static exercises are the exercises that small muscle groups produce relatively bigger power, cause changes to the tension of the muscles and don’t make any changes to the joint movements and length of the muscles (e.g. Weightlifting). For example, long distance running, soccer and tennis require low static and high dynamic exercises; water ski, gymnastics and combat sports require high static and low dynamic exercises; rowing, cycling and boxing require high static and high dynamic exercises [22].
Exercises make hemodynamical and electrophysiological changes to the heart. Dynamic exercises increase oxygen consumption in the muscle mass, cardiac output, heart rate and volume. Thus, systolic blood pressure rises while systemic vascular resistance drops. As a result, minimal changes occur in the average blood pressure. As for static exercises, they cause mild increase in oxygen consumption, cardiac output and heart rate, as well as significant increase in systolic, diastolic and average arterial pressure. Stroke volume and total peripheral resistance don’t change while systemic vascular resistance increases. Both types of exercises increase the requirement for myocardial oxygen. In the cases of abnormal coronary artery and ventricular hypertrophy, ischemia might develop to cause malignant arrhythmia [57].
In the individuals with undiagnosed cardiovascular disease, stress, myocardial ischemia, sympathovagal imbalance and hemodynamical changes might trigger dysrhythmia and cause sudden cardiac death [49,58]. How much sports activities affect individuals regarding sudden cardiac death is related to many variables as the type of sport (such as marathon, triathlon, cycling), intensity of the sport, competitiveness, as well as age, race and genetic characteristics of the individual. Hypertrophic Cardiomyopathy (HCM), coronary artery abnormalities, myocarditis, arrhythmogenic right ventricular dysplasia, ion channel defects and aortic stenosis can all cause etiopathogenesis [46]. Additionally, even though there has been some concerns regarding performance-enhancing substances having serious cardiovascular side-effects that can cause sudden cardiac death, there isn’t any proof presented about it yet [48]. Bonetti et al., after a 2 year study where they observed 20 individuals who were having anabolic steroid treatment or misusing steroids, have shown that the most obvious difference was the decrease in blood HDL and thought the situation could cause atherosclerotic changes and are connected to coronary artery disease [16]. However, new studies are required to determine whether there is a distinct relationship between anabolic steroids, other ergogenic substances and sudden cardiac death.
Death Incidences in Athletes with Hypertrophic Cardiomyopathy Causes
HCM is a genetic condition which is usually found with both mutation in sarcomeric protein-coding genes which is the base of ventricular arrhythmia and left ventricular hypertrophy [44].
First recorded observation of megalocardia in athletes was done by a Swedish clinician named Henschen in 1899 [59]. In 1975, Mongaroth et al. have defined different forms of cardiac hypertrophy among athletes [60]. While the most common sudden cardiac death among athletes under the age of 35 is hypertrophic cardiomyopathy, main cause for sudden cardiac death over the age of 35 is coronary artery disease [44]. Additionally, a Study in United States of America (USA) which analyses death of 1866 young athletes has shown that the HCM is the most common cause for sudden cardiac death [48]. In differently designed epidemiologic studies, similar results were found and prevalence was shown as 1/500 [48,61,62]. This could go up to as high as 1/200 in athletes and most first symptoms could be sudden cardiac death [44,63].
Harmon et al. emphasized that re-evaluation of causes and prevalences of sudden cardiac death could be essential. In this context, National Collegiate Athletic Association (NCAA) has scanned the sudden cardiac deaths in athletes between the years 2003-2013 and detected %25 percent structurally normal heart in these individuals after the autopsies. This shows that rhythm and other conduction disturbances might be the most common etiological factors. Second most common factor is, similar as the earlier studies, coronary artery abnormalities with 11%. However in this study, HCM has dropped behind with 8%, contrary to 30-40% that was found in the earlier studies. Authors indicated that variation of sick population that was included in the studies, as well as no particular agreement on diagnosis of HCM between autopsy experts may cause this variation across the studies [64]. Similarly, a study in England in which 3500 elite athletes were scanned, has detected HCM prevalences as low as 0, 06% [65].
The general opinion about HCM being relatively low-occurring and rarely diagnosed in black population was dramatically changed after it was discovered that 55% of the deaths related to the HCM in USA was in ethnically Afro-American individuals. It was discovered that in Afro-American athletes, the number of post-autopsy diagnosis of HCM was 7 times more than the number of clinically diagnosed athletes. Even though socioeconomic factors such as being able to afford health care services could have a distinct effect on these statistics, misdiagnosis of Hypertension as HCM related left ventricular hypertrophy in asymptomatic population usually by an incorrect approach is considered as another important reason
In conclusion, even though HCM leads the reasons for sudden cardiac death among young athletes, many varying information regarding its prevalence exist. Having routine molecular diagnostic test and cardiovascular pathologists with forensic experts in autopsy applications, is going to detect true prevalence of HCM and thus help us to develop optimal strategies regarding preventing sudden cardiac deaths [44,51].
References
- Myerburg RJ, Castellanos A. Cardiac arrest and sudden cardiac death. Saunders. 2012; 845-884.
- Mendis S, Puska P, Norrving B. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva WHO. 2011.
- Priori SG, Blomstrom-Lundqvist M, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. European Heart Journal. 2015; 36: 2793-2867.
- Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003; 42: 1959-1963.
- Harmon KG, Drezner JA, Wilson MG, Sharma S. Incidence of sudden cardiac death in athletes: a state-of-the-art review. Heart. 2014; 100: 1227-1234.
- Schmied C, Borjesson M. Sudden cardiac death in athletes. J Intern Med. 2014; 275: 93-103.
- Semsarian C, Sweeting J, Ackerman MJ. Sudden cardiac death in athletes. BMJ. 2015.
- Maron BJ. Hypertrophic Cardiomyopathy. Saunders. 2012; 1582-1594.
- Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States,1980-2006. Circulation. 2009; 119: 1085-1092.
- Spirito P, Autore C. Management of hypertrophic cardiomyopathy. BMJ. 2006; 332: 1251-1255.
- Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes III NAM, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 3: Hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies and myocarditis A scientific statement from American Heart Association and American College of Cardiology. Circulation. 2015; 132: 273-280.
- Barbier J, Ville N, Kervio G, Walther G, Carré F. Sports-Specific Features of Athlete’s Heart and their Relation to Echocardiographic Parameters. Herz. 2006; 31: 531-543.
- Rost R, Hollman W. Athlete’s heart-a review of its historical assessment and new aspects. Int J Sports Med. 1983; 4: 147-154.
- Henschen S. Skilanglauf und skiwettlauf: eine medizinische sportstudie. Mitt Med Klin Upsala (Jena). 1899; 2: 15-18.
- Fagard R. Athlete’s Heart. Heart. 2003; 89: 1455-1461.
- Maron BJ, Pelliccia A. The Heart of Trained Athletes Cardiac Remodeling and the Risks of Sports, Including Sudden Death. American Heart Association Circulation. 2006; 114: 1633-1644.
- Mitchell JH, Haskell W, Snell P, Van Camp SP, Maron BJ, Zipes DP, et al. 36th Bethesda Conference: eligibility recommendations for competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2005; 45: 1364-367.
- Hauser AM, Dressendorfer RH, Vos M, Hashimoto T, Gordon S, Timmis GC. Symmetric cardiac enlargement in highly trained endurance athletes: a two-dimensional echocardiographic study. Am Heart J. 1985; 109: 1038-1044.
- Scharf M, Brem MH, Wilhelm M, Schoepf UJ, Uder M, Lell MM. Cardiac magnetic resonance assessment of left and right ventricular morphologic and functional adaptations in professional soccer players. Am Heart J. 2010; 159: 911-918.
- Paterick TE, Gordon T, Spiegel D. Echocardiography: Profiling of the Athlete’s Heart. J Am Soc Echocardiogr. 2014; 27: 340-348.
- Pagourelias ED, Kouidi E, Efthimiadis GK, Deligiannis A, Geleris P, Vassilikos V. Right atrial and ventricular adaptations to training in male Caucasian athletes: an echocardiographic study. J Am Soc Echocardiogr. 2013; 26: 1344-1352.
- Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991; 324: 295-301.
- Bezzina CR, Lahrouchi N, Priori GS. Genetics of Sudden Cardiac death. Circulation Res. 2015.
- Watkins H, Ashrafian H, Redwood C. Inherited cardiomyopathies. N Engl J Med. 2011.
- Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990.
- Vernengo L, Lilienbaum A, Agbulut O, Rodriguez MM. The role of genetics in cardiomyopathy. 2013.
- Richard P, Villard E, Charron P, Isnard R. The genetic bases of cardiomyopathies. J Am Coll Cardiol. 2006.
- Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations and implications for a molecular diagnosis strategy. Circulation. 2003.
- Towbin JA. Inherited cardiomyopathies. Circ J. 2014.
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013.
- Aernout Somsen, Kees Hovingh G, Tulevski I. Familial dilated cardiomyopathy. Clinical Cardiogenetics. Springer. 2011.
- Marcus FI, Edson S, Towbin JA. Genetics of arrhythmogenic right ventricular cardiomyopathy: a practical guide for physicians. J Am Coll Cardiol. 2013.
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999; 97: 175-187.
- Medeiros-Domingo A, Toshihiko Kaku, David J, Tester BS, Pedro Iturralde-Torres, Ajit Itty, et al. SCN4B-encoded sodium channel β4 subunit in congenital long-QT syndrome. Circulation. 2007; 10: 134-142.
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, et al. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004; 119: 19-31.
- Yang Y, Liang B, Liu J, Li J, Grunnet M, Olesen SP, et al. Identification of a Kir3.4 mutation in congenital long QT syndrome. Am J Hum Genet. 2010.
- Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007; 104: 20990-20995.
- Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011; 8: 1308-1339.
- Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004; 109: 30-35.
- Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005; 96: 800-807.
- Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals and sudden cardiac death. Circulation. 2007; 115: 442-449.
- Templin C, Ghadri JR, Rougier JS, Baumer A, Kaplan V, Albesa M, et al. Identification of a novel lossof-function calcium channel gene mutation in short QT syndrome (SQTS6). Eur Heart J. 2011; 32: 1077-1088.
- Crotti L, Marcou CA, Tester DJ, Castelletti S, Giudicessi JR, Torchio M, et al. Spectrum and prevalence of mutations involving BrS1-through BrS12-susceptibility genes in a cohort of unrelated patients referred for Brugada syndrome genetic testing: implications for genetic testing. J Am Coll Cardiol. 2012; 60: 1410-1418.
- Wasfy MM, Hutter AM, Weiner RB. Sudden cardiac death in athletes. Methodist Debakey Cardiovasc J. 2016; 12: 76-80.
- Hoyt WJ, Dean PN, Battle RW. The historical perspective of athletic sudden death. Clin Sports Med. 2015; 34: 571-585.
- Yildiz M. Suggestions on how to do. How to perform the cardiac preparticipation screening in competitive young athletes? Arch Turk Soc Cardiol. 2014; 42: 491-493.
- Yucel AS, Catıkkas F, Gumuşdag H. Sudden deaths ın sports due to cardiovascular reasons. International Refereed Journal of Orthopaedics Traumatology and Sports Medicine 2014; 04: 30.
- Pugh A, Bourke JP, Kunadian V. Sudden cardiac death among competitive adult athletes: a review. Postgrad Med J. 2012; 88: 382-390.
- Akalın F. Sudden death in athletes. Turk Pediatri Ars. 2006; 41: 131-138.
- Germann CA, Perron AD. Sudden cardiac death in athletes: a guide for emergency physicians. Am J Emerg Med. 2005; 23: 504-509.
- Harmon KG, Drezner JA, Wilson MG, Sharma S. Incidence of sudden cardiac death in athletes: a state-of-the-art review. Heart. 2014; 100: 1227-1234.
- Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic and pathological profiles. JAMA. 1996; 276: 199-204.
- Kasikcioglu E. Sudden cardiac death during sports activity. Ital J Pediatr. 2006; 32: 8-11.
- Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol. 1998; 32: 1881-1884.
- Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998; 339: 364-369.
- Corrado D, Basso C, Thiene G. Sudden death in athletes. Lancet. 2005; 366: 547-548.
- Maron BJ, Chaitman BR, Ackerman MJ, Bayés de Luna A, Corrado D, Crosson JE, et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. 2004; 109: 2807-2816.
- Bonetti A, Tirelli F, Catapano A, Dazzi D, Dei Cas A, Solito F, et al. Side effects of anabolic androgenic steroids abuse. Int J Sports Med. 2008; 29: 679-687.
- Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975; 82: 521-524.
- Semsarian C, Maron BJ. Sudden cardiac death in the young, The Med J of Australia. 2002; 176: 149.
- Luong MW, Morrison BN, Lithwick D, Heilbron B. Sudden cardiac death in young competitive athletes. BCMJ. 2016; 58: 138-144.
- Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000; 102: 858-864.
- Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, et al. Incidence, cause and comparative frequency of sudden cardiac death in national collegiate athletic association athletes: a decade in review. Circulation. 2015; 7: 132: 10-19.
- Maron BJ, Carney KP, Lever HM, Lewis JF, Barac I, Casey SA, et al. Relationship of race to sudden cardiac death in competitive athletes with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003; 41: 974-980.
- Basavarajaiah S, Wilson M, Whyte Gregory, Shah A, McKenna, Sharma S. Prevalence of hypertrophic cardiomyopathy in highly trained athletes. J Am Coll Cardiol. 2008; 51: 1033-1039.
