Short Communication
Accumulating data suggest preeclampsia, determined as gestational hypertension with organ failure (e. g. proteinuria), is not a homogenous disease [1-3]. Recently, early- or late-onset types are distinguished according to the gestational week when clinical symptoms first appear. While early-onset preeclampsia (clinical manifestation prior to the 34th gestational week) is thought of a placental disease, most likely due to an immunological imbalance between the mother and her embryo, the late-onset type (clinical manifestation at or following the 34th week) is rather a maternal disease. The pregnancy outcome is much better in the late-onset type.
During normal pregnancy, generalized vasorelaxation allows a cc. 2 l of blood volume augmentation without blood pressure elevation; the sufficient sodium and water retentions are prerequisites for normal fetal development. Taking account the simplified form of the Poiseuille-Hagen equation: Cardiac Output (CO) = (blood) Pressure/ Resistance, pressure increases as either resistance or blood volume (measured as CO) increases. Otherwise, CO is determined as stroke volume x pulse rate.
Classical central hemodynamic examinations showed low CO in preeclamptic patients; fetal growth restriction, secondary to placental insufficiency, has been stated as an ordinary complication [4,5]. However, others reported high CO in preeclampsia [6]. This controversy was further augmented by investigations in which high fetal birth weights were also reported in cases admitted with the diagnosis of preeclampsia [3,7].
Whether early- and late-onset types are conditions in which different maternal answers are given to the same progenitor, or gestational hypertension with organ damage develops in different pathways secondary to different progenitor, remains in question.
The different pathogenesis of gestational hypertension + proteinuria is an attractive theory; it can explain all the controversies found, thus far, in studies with preeclamptic patients. In classical preeclampsia, endothelial dysfunction due to agents originating from an under-perfused placenta, and worsened rheological properties of the blood diminishes the microcirculation [8,9]. Organ dysfunctions (e. g. proteinuria), vasoconstiction, platelet activation, and intrauterine growth restriction with oligohydramnios are reasonable consequences of this under-perfused condition; CO is low in the absence of vascular capacity expansion (Figure 1). These symptoms use to develop prior to the 34th gestational week and quickly worsen, in which this condition could correspond with earlyonset preeclampsia.
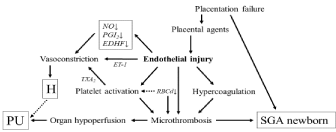
Figure 1: Scheme of the development of early-onset preeclampsia.
NO: Nitric Oxide; PGI2: Prostaglandin I2 (Prostacyclin); EDHF: Endothelium-
Derived Hyperpolarizing Factor; ET-1: Endotheline-1; TXA2: Thromboxane
A2; RBCd: Red Blood Cell Deformability; H: Hypertension; PU: Proteinuria;
SGA: Small for Gestational Age.
If extra sodium and water retention causes gestational hypertension, CO levels should be high. Although, leg/generalized edema can develop earlier, hypertension and mild yet significant proteinuria manifest usually following the 33rd gestational week; this condition may likely correspond with the late-onset type (Figure 2). Due to the good blood supply, fetal somatic development remains normal or even increased for a long time, however, later, due to generalized edema, organ dysfunctions (e.g. proteinuria) can develop and placental abruption could occur. Visible edema, and increased gestational weight gain are characteristic symptoms of late-onset preeclampsia; all of which can be explained by water retention beyond the given vascular capacity. Obese women, as yet with unknown reason, are candidates for developing this overperfused preeclampsia. Proteinuria, a sign of organ failure, can be the consequence of hypertension itself; however, tissue edema may also contribute to the appearance of organ dysfunction.
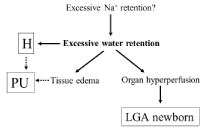
Figure 2: Scheme of the possible development of late-onset preeclampsia
H: Hypertension; PU: Proteinuria; LGA: Large for Gestational age.
Different management strategies in different types of preeclampsia
Currently, pregnancy termination is considered as the only effective management of preeclampsia. This clear maternal interest is frequently limited by fetal interest, since pregnancy prolongation improves pulmonary function of a preterm newborn. Therefore, the decision to prolong or terminate pregnancy in a serious early-onset preeclampsia requires a careful, individual, and a multidisciplinary evaluation. Other efforts thus far failed to effectively improve the maternal or fetal conditions. Aspirin interferes with platelet aggregation and can decrease the incidence of preeclampsia in high-risk cases. However, it fails to improve preeclampsia once the clinical symptoms have already developed [10]. Hypertension is known to worsen endothelial function and, in critical cases, the blood pressure is always high. However, whether hypertension itself leads to catastrophe or hypertension, as in the role of compensating, reflects to the underlying microcirculatory failure in early-onset preeclampsia, has not yet been substantiated. In any case, decreasing blood pressure decreases tissue perfusion, and antihypertensive drugs decrease fetal blood pressure as well. As it is equivocally accepted, gestational hypertension indicates antihypertensive medication when systolic and/or diastolic pressures reach the level of 160 and 110 mm Hg, respectively. Deliberate vasodilator medication is suggested in oral or infusion therapy; during the acute procedure, fetal monitoring is indicated. Extreme high blood pressure requires bolus (2-4 g in 20 min) followed by a maintaining (1-2 g/hour) MgSO4 infusion therapy, as eclampsia prophylaxis.
Measuring CO by impedance cardiography, at an average of 6.8 l/ min was found in normal third-trimester patients [11]. In preeclamptic patients we detected a markedly lower but also a strikingly higher level of CO. Enrolling preeclamptic patients with a CO > 7.48 l/min, all, which proved to be late-onset types since the clinical symptoms appeared following the 33rd gestational week. If high blood volume is responsible for hypertension and edema, the use of diuretics appears to be reasonable and justified. In such cases we used 40 mg of oral furosemide to evaluate the effect of blood volume decrease on blood pressure. A single dose of furosemide resulted in a parallel decrease of CO and blood pressure levels. Pulse rate remained stable, suggesting the CO decrease was due to stroke volume decrease, secondary to decrease of blood volume. It is worth mentioning, neither maternal nor fetal side effects were noticed [12].
This preliminary study suggests the possible usefulness of diuretics in cases of gestational hypertension or preeclampsia when the level of CO is high, patients experience generalized edema, and the fetal condition is normal. On the other hand, these results suggest extreme water retention can play a crucial role in the development of hypervolemic gestational hypertension or late-onset preeclampsia.
References
- Phillips JK, Janowiak M, Badger GJ, Bernstein IM. Evidence for distinct preterm and term phenotypes of preeclampsia. J Matern Fetal Neonatal Med. 2010; 23: 622-626.
- Myatt L, Roberts JM. Preeclampsia: Syndrome or disease? Curr Hypertens Rep. 2015; 17: 83-90.
- Vatten LJ, Skjaerven R. Is pre- eclampsia more than one disease? BJOG Int J Obstet Gynaecol. 2004; 111: 298-302.
- Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002; 287: 1383-1386.
- Visser W, Wallenburg HCS. Central hemodynamic observations in untreated preeclamptic patients. Hypertension. 1991; 17: 1072-1077.
- Easterlin TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol. 1990; 76: 1061-1069.
- Rasmussen S, Irgens LM. Fetal growth and body proportion in preeclampsia. Obstet Gynecol. 2003; 101: 575-583.
- O’Brien M, Baczyk D, Kingdom JC. Endothelial dysfunction in severe preeclampsia is mediated by soluble factors, rather than extracellular vesicles. Sci Rep. 2017.
- Schauf B, Mannschreck B, Becker S, Dietz K, Wallwiener D, Aydeniz B. Evaluation of red blood cell deformability and uterine blood flow in pregnant women with preeclampsia or iugr and reduced uterine blood flow following the intravenous application of magnesium. Hypertens Pregnancy. 2004; 23: 331-343.
- Moore GS, Allshouse AA, Post AL, Galan HL, Heybrone KD. Early initiation of low-dose aspirin for reduction in preeclampsia risk in high-risk women: a secondary analysis of the MFMU High-Risk Aspirin Study. J Perinatol. 2015; 13: 328-331.
- Tamás P, Szilágyi A, Jeges S, Vizer M, Csermely T, Ifi Z, Bálint A, Szabó I. Effects of maternal central hemodynamics on fetal heart rate patterns. Acta Obstet Gynecol Scand. 2007; 86: 711-714.
- Tamás P, Hantosi E, Ifi Z, Farkas B, Betlehem J, Bódis J. Preliminary study of the effects of furosemide on blood pressure during late-onset pre-eclampsiain in patients with high cardiac output. Int J Gynecol Obstet. 2017; 137: 87-90.
