
Editorial
Austin Immunol. 2016; 1(1): 1001.
NK Cell Responses to Influenza Virus are Affected by Host Genetics and Infecting Viral Doses
Kai Zhou¹, Jing Wang1,2 and Min Fang¹*
¹CAS Key Laboratory of Pathogenic Microbiology and Immunology, Chinese Academy of Sciences, China
²Department of Immunology, University of Chinese Academy of Sciences, China
*Corresponding author: Min Fang, CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China
Received: May 19, 2016; Accepted: May 20, 2016; Published: May 23, 2016
Editorial
In recent years, frequently emerging Influenza A Virus (IAV) epidemics have become a challenge to both veterinary and public health worldwide, such as the 2009 pandemic influenza A H1N1, avian influenza A H5N1, and H7N9 [1]. Influenza symptoms vary from mild disease to death; variations in influenza-induced disease severity in humans are usually attributed to the virulence of different influenza virus strains or the age or immune statues of infected individuals [2-4]. However, the role of genetically determined host factors is less well understood, and only recently, IFITM3 has been identified to restrict the morbidity and mortality associated with influenza in human population [5]. The mouse has been shown to represent a particularly useful model to study the virulence of IAV infection, and previous studies have shown that the genetic background of mouse strains strongly influences the prognosis and severity after IAV infection [2,4]. However, how the components of the host immune system, especially lymphocytes affecting on or contributing to the genetic related resistance or susceptibility after IAV infection are not known.
NK cells are large granular lymphocytes that mediate innate protection from some viral infections and tumor cells [6,7]. NK cells are essential for resistance to several viral infections, such as Epstein-Barr virus, human cytomegalovirus, Varicella-zoster virus and herpes simplex virus [8-11]. The roles that NK cells play during influenza virus infection are intricate. Several studies highlight the pivotal role of NK cells in the control of IAV infection in that defect in NK cell activity or depletion of NK cells result in delayed viral clearance and increased morbidity and mortality [12-14]. However, there are also examples in which NK cells exacerbate morbidity and pathology during lethal dose influenza virus infection in mice [15- 17], which indicate that NK cells may play dual roles during influenza virus infection in mice, conferring beneficial or deleterious function depending on the viral dose. Furthermore, production of IL-22 by NK and NKT cells has an important role in the repair of epithelial damage caused by IAV [17]. Our recent studies have shown that NK cells are also involved in the thymic atrophy during IAV infection [18]. Meanwhile, NK cells play important roles in bridging the innate and adaptive immune responses to IAV. The major and complex roles of NK cells in IAV infection still require further in-depth investigations.
Previous studies show that host genetic background strongly influences the responses to IAV infection [4]. However, the functions of NK cells during IAV infection with regard to different host genetic backgrounds remained unclear. We recently found that NK cells play differential roles against IAV infection, depending on the host genetic backgrounds [19]. In order to investigate whether NK cells contribute to the genetic related resistance or susceptibility in different inbred mouse strains, we depleted NK cells in six inbred mouse strains, including C57BL/6, BALB/c, C3H, DBA/2, FVB and 129 mice. Surprisingly, NK cells played an important protective role only in 129 mice during high-dose IAV infection. NK cell depletion resulted in significantly increased virus titers in 129 mice, but not in the other five mouse strains. Further mechanistic studies revealed that after high dose IAV infection, NK cells in 129 mice quickly activated and accumulated to the infected lungs compared to that of C57BL/6 mice at 1 day post infection (dpi). The swift and strong NK cell responses efficiently controlled early pulmonary viral replication in 129 mice, providing survival privilege. We also identified that the early activation of TLRs and RIG-I signaling at 1 dpi in 129 mice resulted in quick production of type 1 interferon and inflammatory cytokines, which are important reasons for the swift kinetics of NK cell responses in 129 mice following high-dose IAV infection. Furthermore, the early NK cell accumulation in 129 mice was resulted from CCR2-dependent NK cell migration, not because of NK cell proliferation.
With 18 hemagglutinin subtypes and 11 neuraminidase subtypes, influenza A virus includes thousands of strains, and some of them are responsible for flu outbreak in recent years [20-22]. We further measured NK cell responses to high-dose H3N2 virus infection. As the same with PR8 infection, NK cell responses to high-dose H3N2 virus infection were also earlier in 129 mice compared to that of C57BL/6 mice, but the overall magnitude was much lower compared to that of PR8 virus, which indicates that NK cell responses were also affected by virus strains.
Besides genetic backgrounds, the infecting virus doses were also important to NK cell responses in mice. NK cells were not protective against low-dose IAV infection both in 129 mice and C57BL/6 mice. The viral replication was also not affected by NK cell-depletion in both mice. By measuring the NK cell responses and also the cytokine profiles in both mouse strains, we found that the kinetics of NK cell responses were similar between 129 mice and C57BL/6 mice. Further, no early cytokine production was found in both mouse strains at 1 dpi [19].
Thus, as shown in (Figure 1), our data demonstrated that the responses and anti-viral functions of NK cells are affected by the host genetic backgrounds, and also the infecting viral doses. In 129 mice, the early induction of type 1 IFNs and inflammatory cytokines are important reasons for the strong and prompt NK cell responses. This work links the kinetics and magnitudes of NK cell responses to the differential roles NK cells play during IAV infection. This finding also indicates that NK cell responses might be an important indicator for their function during viral infections, as NK cells are essential for some viral infections, while are dispensable during certain viral infections.
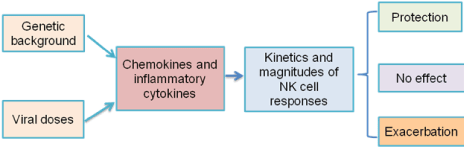
Figure 1: NK Cell function are affected by host genetic background and
infecting viral dose.
Three main factors are involved in determining the variability in the severity of influenza virus infection: the intrinsic pathogenicity of the virus, acquired host factors (such as immunity and comorbidity), and intrinsic host susceptibility. This demonstration that the host genetic backgrounds fundamentally affect the kinetics and magnitudes of NK cell responses, thus related NK cell functions during IAV infection is unique. Further investigation for the molecular mechanisms that result in the differential activation of the pattern recognition receptors in 129 and C57BL/6 mice might identify new genetic related restriction factors for severe influenza infection.
References
- Webster RG, Govorkova EA. Continuing challenges in influenza. Annals of the New York Academy Sciences. 2014; 1323: 115-139.
- Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nature Communications. 2014; 5: 3864.
- Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Scientific Reports. 2016; 6: 25051.
- Srivastava B, Blazejewska P, Hessmann M, Bruder D, Geffers R, Mauel S, et al. Host genetic background strongly influences the response to influenza a virus infections. PLoS One. 2009; 4: 4857.
- Everitt AR, Clare S, Pertel T, John SP, Wash RS, Smith SE, et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012; 484: 519-523.
- French AR, Yokoyama WM. Natural killer cells and viral infections. Current Opinion in Immunology. 2003; 15: 45-51.
- Wu J, Lanier LL. Natural killer cells and cancer. Advances in Cancer Research. 2003; 90: 127-56.
- Kunder SC, Wu L, Morahan PS. Role of NK cells in immunomodulatormediated resistance to herpes virus infection. Antiviral Research. 1993; 21: 103-118.
- Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes and Infection. 2002; 4: 1545-1558.
- Bukowski JF, Woda BA, Welsh RM. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. Journal of Virology. 1984; 52: 119-128.
- Lanier LL. Evolutionary struggles between NK cells and viruses. Nature Reviews Immunology. 2008; 8: 259-268.
- Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nature Immunology. 2006; 7: 517-523.
- Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mechanisms of Ageing Development. 2008; 129: 223-230.
- Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. Journal of Immunology. 1986; 136: 1435-1441.
- Zhou G, Juang SW, Kane KP. NK cells exacerbate the pathology of influenza virus infection in mice. European Journal of Immunology. 2013; 43: 929-938.
- Abdul-Careem MF, Mian MF, Yue G, Gillgrass A, Chenoweth MJ, Barra NG, et al. Critical role of natural killer cells in lung immunopathology during influenza infection in mice. Journal of Infectious Diseases. 2012; 206: 167- 177.
- Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunology. 2013; 6: 69-82.
- Duan X, Lu J, Zhou K, Wang J, Wu J, Gao GF, et al. NK-cells are involved in thymic atrophy induced by influenza A virus infection. Journal of General Virology. 2015; 96: 3223-3235.
- Zhou K, Wang J, Li A, Zhao W, Wang D, Zhang W, et al. Swift and Strong NK Cell Responses Protect 129 Mice against High-Dose Influenza Virus Infection. Journal of Immunology. 2016; 196: 1842-1854.
- Liu J, Xiao H, Lei F, Zhu Q, Qin K, Zhang XW, et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science. 2005; 309: 1206.
- Munster VJ, De Wit E, Van Den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, et al. Pathogenesis and transmission of swine-origin 2009 A (H1N1) influenza virus in ferrets. Science. 2009; 325: 481-483.
- Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature. 2013; 501: 551-555.