
Review Article
Austin Immunol. 2016; 1(1): 1004.
Hypotheses about the Potential Role of Mesenchymal Stem Cell on Nasal Polyposis: A Soft Inflamed Tissue Suffering from Mechanical Dysfunction
Pezato R1,2*, Gregorio LC¹, Voegels RL², Kosugi EM¹, Pinna F², Perez-Novo C4, Bezerra TF², De Almeida DC³ and De Camargo-Kosugi CM¹
¹Department of Otolaryngology-Head and Neck Surgery, Federal University of Sao Paulo, Brazil
²Department of Otolaryngology and Ophthalmology, University of Sao Paulo, Brazil
³Department of Medicine, Federal University of Sao Paulo, Brazil
4Department of Biomedical Sciences, University of Antwerp, Belgium
*Corresponding author: Rogerio Pezato, Otonis Street, 700 Top Floor, Vila Clementino, 04025-002 Sao Paulo, SP, Brazil
Received: June 16, 2016; Accepted: July 29, 2016; Published: August 05, 2016
Abstract
Background: Nasal Polyposis is a chronic inflammatory condition of the upper airway, occurring in 1-4% of the general population. Nasal Polyposis is characterized by a severe chronic inflammatory process and an abnormal remodeling implicated in a tissue mechanical dysfunction.
Principle: In this review we describe the mechanism used by stem cells to interact with inflammatory cells and during the healing process, we also evaluate the impact of mesenchymal stem cell on Nasal Polyposis.
Results: In this review we demonstrate the potential role of mesenchymal stem cell on Nasal Polyposis disease, a cell with immunoregulatory properties and modulation on healing process.
Conclusion: mesenchymal stem cell has immunoregulatory properties on Nasal Polyp tissue and we speculate a potential role on the remodeling process.
Keywords: Mesenchymal stem cell; Nasal polyposis; Stem cell; Chronic rhinosinusitis; Sinus
Introduction
Nasal polyposis is a chronic inflammatory condition of the upper airway characterized histologically by the infiltration of inflammatory cells like eosinophils or neutrophils and occur in 1-4% of the general population [1,2]. Clinically, nasal polyposis presents nasal obstruction, hyposmia, rhinorrhea and reduced quality of life and nasal endoscopic examination confirms polyp formation from middle meatus. Nasal Polyps (NP) are inflammatory outgrowth of paranasal sinus mucosa, most often benign, frequently bilateral, and typically develop in adulthood, and characterized by inflammatory cell infiltration and abnormal tissue remodeling [3,4].
Two major factors are related to nasal polyp formation: an abnormal remodeling response and a lack of immunoregulatory effects, creating an imbalance in immunomodulation and, consequently, favoring inflammation.
Stem cells
In 1993, Tissue Engineering, a new field, was approached by Langer and Vacanti. They proposed to apply the principles of biology and engineering to develop biological substitutes to restore the function of injured tissues and organs. However, the authors described many critical points to reach the success of the cell/tissue transplantation and maintenance [5]. After that, tissue engineering has advanced considerably.
Cell therapy and tissue engineering play an important role on lesions restoring of dysfunctional or injured tissues [6]. Both adult and embryonic stem cells have emerged as a promise and innovative tools to regenerate organs and tissues, as well as, heart conditions, liver injury, diabetes, leukemia, bone regeneration, muscle regeneration in progressive muscular dystrophies, and others diseases [7,8].
Stem cells are characterized by a population of undifferentiated cells with the capacity to extensively proliferate (self-renewal). Usually arise from a single cell (clonal), and differentiate into several types of cells and tissues (potency). There are several sources of stem cells with varying potencies. The Embryonic Stem Cells (ESC) has more differentiation and expansion potential than adult stem cells, but that is a concern about ethical and religious beliefs that, consequently, limit the research with ESC [7-9].
The Mesenchymal Stem Cells (MSC) are multipotent cells with ability to differentiate into several mesenchymal cell lines (chondrocytes, adipocytes, and osteocytes) that can be obtained from several adult and fetal tissues, and do not carry ethical concerns such as ESC, but are more limited in terms of expansion and differentiation capabilities [7,10-13].
Mesenchymal stem cell
MSCs possess a great therapeutic potential, such as immunosuppressive effects and homing capacity to sites damages.
The best-documented cytokines secreted by MSCs are TGF-β1, PGE-2, IDO, IL-10, IL-6, MMP-2, MMP-9, TIMP-2, TIMP-3, nitric oxide, chemokine ligands 2 and 5, Human Leukocyte Antigen (HLA)-5, heme oxygenase-1, hepatocyte growth factor, and leukemia inhibitory factor [14].
More than a direct cellular therapy, MSCs may be a very useful adjunct for investigation of the inflammatory process in NP. MSCmediated immunomodulation can occur via. cell-to-cell contact or by release of soluble factors.
It is essential for any NP treatment to soothe the inflammatory process in nasal tissue and blunt the imbalanced Th response. Modulating the immune response in NP to favour anti-inflammatory mediators and reduce exacerbated Th2 expression can change the course of the disease. Another crucial point in NP treatment is improving the healing process by increasing the quantity and quality of ECM.
Inflammation in NP
In NP, the inflammatory cells are globally increased and so their corresponding mediators, such as Immunoglobulin (Ig) E, Eosinophilic Cationic Protein (ECP), Interferon (IFN)-γ, Myeloperodixase (MPO), indoleamine 2, 3-dioxygenase (IDO), RANTES, Granulocyte/Macrophage Colony-Stimulating Factor (GM-CSF), eotaxin, interleukin (IL)-4, IL-5, IL-6, IL-12, IL-17, Tumor Necrosis Factor (TNF)-a are also increased [15-17]. In contrast, there is not a proper immunoregulatory response to counterbalance the storm of inflammatory mediators. NP presents a decrease of Treg cells [18], moreover, their migration potential is impaired too [19]. TGF-β is an important mediator of the healing process. It is implicated in the immunoregulation, being a key role on naïve T cell differentiation to Treg [20].
The imbalance between pro-inflammatory and anti-inflammatory mediators in NP is also found at the eicosanoid pathway. Leukotrienes, potent inducers of airway inflammation, are higher expressed in nasal polyp tissue when compared with normal mucosa. In contrast, Prostaglandin (PG) E2, a PG with anti-inflammatory properties, is found to be decreased in nasal polyp when compared with normal nasal mucosa [21]. Although the imbalance in the eicosanoid pathway is the hallmark of AERD, it is not limited to this disease, being found in NP without aspirin sensitivity [21,22].
As well as the increase of inflammatory cells impacts on the release of inflammatory mediators, these cytokines in its turn alter the expression, migration, activation, survival and apoptosis of these cells.
Several theories regarding the development and pathogenesis of the disease have been proposed, such as the role of epithelial barrier function [23-28], innate and adaptive immunity [29-38], eosinophilic inflammation [39-40], genetic factors [41-47], as well as proteins profile, including cell cycle and apoptosis proteins [48- 55].
Many studies try to explain this severe inflammatory environment. Li and colleagues reported that a down-regulation of Activator Protein 1 (AP1) and its related genes (COX2, IL-6, and epidermal growth factors) were associated with damage of epithelial structure, while up-regulation of p63 in basal cells was implicated in the epithelial hyperplasia in NP [56].
Some authors evaluated the IL-6 gene polymorphism (rs1800795) in NP and asthma. Inflammatory group presented higher percentage of the variant genotype when compared to the control group, leading us to believe that NP is a multifactorial disease where genetic could play a role [42].
Antigen presenting cells such as Dendritic Cells (DC) are the responsible for activation, maturation and differentiation of naïve T cells, with two distinct subsets: plasmacytoid (pDC) and myeloid Dendritic Cells (mDC). T helper cell differentiation is mediated by epigenetic processes [57]. It was demonstrated an altered pDC/mDC balance in nasal polyp tissue, pDCs seem to be more susceptible to an inflammatory cytokine milieu, decreasing in more inflamed environment [58].
NP was long characterized as a disease orchestrated by Th2 cells [59], and eosinophils were long considered the main cells found in nasal polyp tissue, with high levels of related mediators, such as IL-5 and ECP. Other pro-inflammatory molecules, including chemokines, IgE, and lipid metabolites, were part of the list of the main mediators involved in NP [60].
Based on the knowledge that NP was a Th2-driven disease, without strong genetic marker, and considering that an effective animal model were lacking in nasal polyposis, several studies aimed to identify causative mechanisms for this Th2 cell-polarized immune response. The importance of staphylococcal superantigens as a disease modifier favoring an exacerbated Th2 response has been already demonstrated [61]. Fungal antigens have also been demonstrated to secrete Th2 cytokines [62]. Furthermore, a group of epithelial cell cytokines, such as IL-25, IL-33, and TLSP, has been implicated in Th2 skewing [63-64].
In 2008, Zhang et al. provided one of the most valuable findings in the history of NP research by breaking the paradigm that NP is only characterized by Th2-driven inflammation. The authors demonstrated a predominance of neutrophils and a high influence of Th1/Th17 cells in the Chinese population [65]. A storm of subsequent studies conducted in Asia have ratified these results [66].
More accurate than typifying a Th drive disease should be the imbalance in the T cells responsible for the NP inflammation. T helper cells co-expression was found in all samples of NP especially in the Th17 cell population which produced not only IL-17, but also IFNγ and IL-22 [67]. Th2 cells were rarely found in healthy nasal mucosa or in Chronic Rhinosinusitis (CRS) without NP, but they were present in NP expressing high variability of cytokine. Noteworthy, Derycke, et al. [67] did not observe Th2 cells producing IL-17 or IFNγ.
Although a mix of T cell subtypes takes part in the nasal polyp inflammatory process, there is a clear association between the number of Th2 cells with IL-5, ECP, IgE levels, severity of symptoms and responsiveness to treatment, creating a specific endotype [68]. The stereotype of this endotype is found in Aspirin Exacerbated Respiratory Disease (AERD). Considering that CRS could be a disease with a great spectrum, or even different diseases with the same name, nasal polyposis represents the most severe form of upper airway inflammation, especially in combination with Aspirin-Induced Asthma (AIA) [69].
Until now, nasal polyposis is a challenging disease. The goal of CRS treatment is to achieve and maintain clinical control, in which the patients do not have symptoms or the symptoms are not bothersome [2]. The majority of treatment strategies include sinus surgery, intranasal and systemic corticosteroids and antibiotics. A large variety of new different treatments have been studied including anti-IgE, anti-IL5, antimycotics, antihistamines, imunossupressants, furosemide, leukotriene antagonist, aspirin desensitize, capsaicin and others. However, it remains unknown why some patients with CRS develop NP and others do not, and why some patients have difficultto- threat CRS.
In this context, it is imperative to investigate immunomodulatory mechanisms in NP and the reasons behind their failure to diminish inflammation in this setting.
Immunoregulatory effects of MSC in NP
There are recent studies, in vitro, assessing the effects of MSC in nasal polyposis. The results are promisors and encourage us to better investigate its role on NP. In 2014, for the first time, the effects of bone marrow-derived MSCs on nasal polyp tissue were studied [12]. For this purpose, nasal polyp tissue samples from 12 patients with an established diagnosis of NP were cultured with and without MSC co-culture. Nasal polyp-derived cells from fresh tissue consisted of a variety of inflammatory cells, such as B cells, natural killer cells, monocytes, dendritic cells, and Th lymphocytes. A significant decrease in the frequency of these cells and an increase in the frequency of Treg cells were observed after co-culture with MSCs. Simultaneously, the presence of MSCs inhibited CD4+ and CD8+ T-cell proliferation, and changed the global cytokine profile from an pro-inflammatory to an anti-inflammatory response, increasing IL-10 and decreasing IL-2, TNF-a and IFN-γ expression. These data demonstrated the immunoregulatory effects of MSCs on the nasal polyp microenvironment in vitro. These results were confirmed by Cho, et al. in 2014 [70].
In 2015, mesenchymal stem cells were isolated from nasal polyps contributing to a better understanding of the role of these cells on NP [71].
We highlight the inability of nasal polyp tissue to produce PGE2, IL-10 and TGF- β1. It would be of interest to unveil the mechanisms that underlie the increase in the concentrations of these mediators in presence of MSC.
The next step for MSC therapy in nasal polyposis is to investigate if nasal polyp-derived MSCs have the same properties found in bone marrow-derived mesenchymal stem cells.
Recent studies, not yet published, suggest that nasal polypderived MSCs exhibit a distinct gene expression profile from bone marrow-derived MSCs.
Tissue remodeling
Rather than a typified inflammatory disease, CRSwNP characterizes by impairment in the remodeling process. The remodeling process warrants a closer attention due to marked differences between CRS with and without NP. The nasal polyp histology is characterized by diffuse mucosal oedema with imbalance in the Extracellular Matrix (ECM) deposition, unlike chronic rhinosinusitis without nasal polyposis where is found fibrosis [72].
ECM is formed by proteoglycan polysaccharide (heparin sulfate, keratin sulfate, condroitin sulfate), Non-proteoglycan polysaccharide (hyaluronic acid), fiber (elastin, collagen) and others (fibronectin, laminin). The combination among these elements will be responsible for tissue stiffness and elasticity.
Studies involving extracellular matrix in CRSwNP have demonstrated a lack of collagen in nasal polyps when compared to those in healthy subjects [73] the same results were found for proteoglicans biglycan, lumican [74] tenascin and fibronectin [75].
More than a lack of ECM at all, in CRSwNP the extracellular matrix is formed by altered composition in terms of quantity and quality of the elements involved in the ECM formation (Figure 1). For instance, hyaluronan of small size and hyaluronidase are found increased in nasal polyp, suggesting that degradation of hyaluronan occurred through the action of hyaluronidase, creating small fragments of hyaluronan, a potent inflammatory mediator [76]. Another example of this imbalance in the extracellular formation lies on the increased deposition of fibrin in nasal polypoid tissue due the overproduction of factor XIII-A [77].
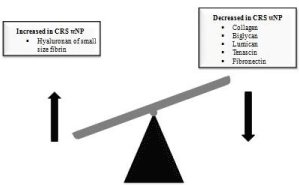
Figure 1: Altered deposition of extracellular matrix in nasal polyp tissue.
Transforming Growth Factor beta-1 (TGF-β1) is implicated as a key protein in the tissue remodeling process; it stimulates fibrosis (by attracting stromal cells), angiogenesis, and accumulation of extracellular matrix. In NP, expression of TGF-β1 is lower than in CRS without NP and in healthy controls [15,78-79]. Matrix Metalloproteinase (MMP)-7 and MMP-9 levels are increased, whereas levels of Tissue Inhibitor of Metalloproteinases (TIMP)-1 and TGF- β1-activated PAI-1 (Plasminogen Activator Inhibitor-1) are decreased when compared with normal nasal mucosa [74,80]. Evaluation by immunohistochemistry demonstrated different concentrations of metalloproteases (MMP-1, MMP-2, MMP-7, and MMP-9) in CRSwNP mucosa when compared with healthy nasal mucosa. According to the different tissue structures (epithelium, glands, vessels, and ECM, de [81]).
This imbalance can be partly explained by the lack of TGF- β1 in NP and its inhibitory effect on MMP-9 and plasminogen activator via. TIMP-1 and PAI-1, respectively [74,80].
The impairment of ECM in NP patients creates a mechanical dysfunction in their nasal mucosa [82]. The balance between hydrostatic and oncotic pressure are responsible for limiting the edema formation during the inflammatory process. In NP, the interstitial hydrostatic pressure does not increase properly in response of the influx of water [83] and protein from the capillary to the nasal polyp tissue during inflammation, hindering the return of water to the capillary, and consequently favoring edema formation (Figure 2). The failure in adequately increase the interstitial hydrostatic pressure in NP could be partly explained by the unappropriated formed connective tissue in NP.
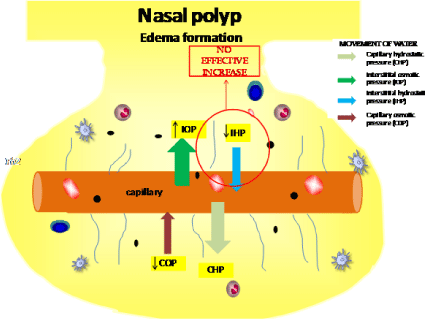
Figure 2: Illustration of movement of water according to pressure influence during chronic inflammation in nasal polyp, favoring edema formation due an ineffective
increase of interstitial hydrostatic pressure.
Therapeutic potential of MSC on fibrosis modulation
Therapeutically, the Mesenchymal Stem Cells (MSCs) produce a broad variety of cytokines, chemokines and growth factors, which are potentially associated with tissue repair and regeneration [84]. In light of this evidences, MSCs possess a great trophic multipotentiality representing an innovative and affordable treatment for acute and chronic diseases [85]. However, the precise participation of MSCs in the fibrotic process is still unclear. Based on set of evidences, it is suggested that MSCs can be useful to modulate the fibrotic process.
In this sense, a pioneering study using a kidney disease model revealed that animals treated with MSCs presented reduction in the potential indicators of renal fibrosis (e.g. low a-SMA index) [86]. After, an elegant work reported that MSCs can effectively attenuate renal fibrosis through tissue remodelling and immunosuppressive activity [87]. Further, the same group demonstrated using an experimental model of interstitial fibrosis that MSC treatment promoted a substantial reduction in the fibrotic-related molecules levels (e.g. collagen-1, vimentin and FSP-1) [88].
To investigate the MSCs anti-fibrotic effect in chronic process, two studies using different models of chronic kidney disease (diabetic nephropathy and chronic aristolochic acid nephropathy) showed that MSC therapy effectively prevented the renal injury (e.g. low creatinine and urea levels) and promoted a decreasing in the fibrosis markers (e.g. collagen, TGF-β and a-SMA) concomitant to enhancement of renal protective molecules (e.g. HGF, E-cadherin and BMP-7) [88- 90]. In addition, using a murine full-thickness skin wounds model, the authors identified that MSC-released TSG-6 can improve wound healing process by limiting Mf activation, inflammation and further fibrosis involvement [91]. Moreover, also have been reported some beneficial effects of MSC treatment for liver fibrosis. In a rat model of dimethylnitrosamine-induced liver fibrosis, the use of HGF-secreting MSCs produced more significant reduction in collagen levels and fibrotic score, enhancing hepatocyte function [92].
To understand the MSC anti-fibrotic mechanism some studies have suggested that MSCs may produce matrix-remodelling molecules, which will recast the affected area, promoting functional improvement. In this context, MSCs can secrete distinct Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of Metalloproteinases (TIMPs), which can infer a synergistic effect on fibrotic remodelling process. Hence, the appropriate balance between MMPs and TIMPs may determine the functional recovery of the fibrotic affected area [93-95].
In fact, experimental models of infarcted hearts demonstrated that the TIMP2/MMP2 and TIMP3/MMP9 ratios can be altered after MSC treatment [96]. Additionally, cardiac fibroblasts cultivated with MSCs conditioned medium showed a reduction in the collagen secretion and an increasing in the MMP2, MMP-9 and MT1-MMP levels. After, when MSCs were injected in rat with post-ischemic heart failure, a significant decreasing in ventricular fibrosis and an improvement in cardiac function were observed [97]. Another study using MSC for treating liver fibrosis also reported a decreasing in MMP-9, MMP-13, MMP-14 and TIMP-1 expression, after MSCs infusion [92].
Based on these experimental findings, the MSCs through use of proteolytic function of MMPs and its molecular regulators TIMPs, may potentiality modulate the MMPs/TIMPs balance to remodel the fibrotic area and recover tissue functionality.
Impairment of the remodeling process is a cornerstone of NP pathophysiology. Altered deposition of extracellular matrix contributes to the formation of soft tissue, and impairs the interstitial hydrostatic pressure response, increasing edema. This imbalance in ECM production is partially explained by a low expression of TGF-β1 and TIMP [73,74]. Thus, it would be extremely useful to investigate the mechanisms involved in release of these mediators in the presence of MSC and their impact on the remodeling process in nasal polyposis
We conclude emphasizing the importance of further studies involving MSCs in nasal polyposis due to the dual therapeutic properties of these cells (immunoregulation and healing modulation) to weaken the pillars of nasal polyposis: impaired remodeling process and severe chronic inflammation.
Authorship Contribution
All authors contribute significantly for this review.
Rogerio Pezato wrote and revised the text
Richard Louis Voegels, Luiz Carlos Gregorio revised the text.
Eduardo Macoto Kosugi, Fabio Pinna, Claudina Perez-Novo, Thiago F Bezerra, literature survey and relevant opinions and insights.
Danilo Candido de Almeida, Cintia Meirelles de Camargo- Kosugi wrote about MSC and stem cell properties, respectively.
References
- Pawankar R, Nonaka M. Inflammatory mechanisms and remodeling in chronic rhinosinusitis and nasal polyps. Curr Allergy Asthma Rep. 2007; 7: 202–208.
- Fokkens W J, Lund V J, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps. Rhinol Suppl. 2012; 3: 1–298.
- Stevens WW, Schleimer RP, Chandra RK, Peters AT. Biology of nasal polyposis. J Allergy Clin Immunol. 2014; 133: 1503.
- Yu XM, Li CW, Chao SS, Li YY, Yan Y, Zha XN, et al. Reduced growth and proliferation dynamics of nasal epithelial stem/progenitor cells in nasal polyps in vitro. Sci Rep. 2004; 4: 4619.
- Langer R, Vacanti JP. Tissue engineering. Science. 1993; 260: 920–926.
- Tran C, Damaser MS. The potential role of stem cells in the treatment of urinary incontinence. Ther Adv Urol. 2014; 7: 22–40.
- Pirraco RP, Reis RL. Tissue Engineering: New Tools for Old Problems. Stem Cell Rev Reports. 2013; 11: 373-375.
- Zatz M. Stem cell researches in Brazil: present and future challenges. Stem Cell Rev. 2009; 5: 123–129.
- Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013; 85: 3–10.
- Secco M, Moreira YB, Zucconi E, Vieira NM, Jazedje T, Muotri AR, et al. Gene expression profile of mesenchymal stem cells from paired umbilical cord units: cord is different from blood. Stem Cell Ver. 2009; 5: 387–401.
- Secco M, Zucconi E, Vieira NM, Fogaca LL, Cerqueira A, Carvalho MD, et al. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008; 26: 146–150.
- Pezato R, Almeida DC, Bezerra TF, De Sa Silva F, Perez-Novo C, Gregorio LC, et al. Immunoregulatory effects of bone marrow-derived mesenchymal stem cells in the nasal polyp microenvironment. Mediators Inflamm. 2014; 583409.
- Jazedje T, Bueno DF, Almada BVP, Caetano H, Czeresnia CE, Perin PM, et al. Human Fallopian Tube Mesenchymal Stromal Cells Enhance Bone Regeneration in a Xenotransplanted Model. Stem Cell Rev Reports. 2012; 8: 355–362.
- Bassi EJ, De Almeida DC, Moraes-Vieira PM, Camara NO. Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev. 2012; 8: 329-342.
- Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000; 14: 279-290.
- Bachert C, Gevaert P, Holtappels G, Van Cauwenberge P. Mediators in nasal polyposis. Curr Allergy Asthma Rep. 2002; 2: 481-487.
- Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope. 2013; 123: 72-78.
- Roongrotwattanasiri K, Pawankar R, Kimura S, Mori S, Nonaka M, Yagi T. Decreased Expression of FOXP3 in Nasal Polyposis. Allergy Asthma Immunol Res. 2012; 4: 24-30.
- Kim YM, Munoz A, Hwang PH, Nadeau KC. Migration of regulatory T cells toward airway epithelial cells is impaired in chronic rhinosinusitis with nasal polyposis. Clin Immunol. 2010; 137: 111-121.
- Liu XJ, Zhang JF, Sun B, Peng HS, Kong QF, Bai SS, et al. Reciprocal effect of mesenchymal stem cell on experimental autoimmune encephalomyelitis is mediated by transforming growth factor-beta and interleukin-6. Clin Exp Immunol. 2009; 158: 37-44.
- Perez-Novo CA, Watelet JB, Claeys C, Van Cauwenberge P, Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005; 115: 1189-1196.
- Pezato R, Swierczynska-Kr?pa M, Nizankowska-Mogilnicka E, Derycke L, Bachert C, Perez-Novo CA. Role of imbalance of eicosanoid pathways and staphylococcal superantigens in chronic rhinosinusitis. Allergy Eur J Allergy Clin Immunol. 2012; 67: 1347–1356.
- Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perpectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinology. 2008; 22: 549-559.
- Fruth K, Goebel G, Koutsimpelas D, et al. Low SPINK5 expression in chronic rhinosinusitis. Laryngoscope. 2012; 122: 1198–1204.
- Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012; 130: 1087–1096.
- Shikani AH, Sidhaye VK, Basaraba RJ, Shikani HJ, Alqudah MA, Kirk N, et al. Mucosal expression of aquaporin 5 and epithelial barrier proteins in chronic rhinosinusitis with and without nasal polyps. Am J Otolaryngol 2014; 35: 377–383.
- Wise SK, Laury AM, Katz EH, Den Beste KA, Parkos CA, Nusrat A. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int Forum Allergy Rhinol. 2014; 4: 361-370.
- Pothoven KL, Norton JE, Hulse KE, Suh LA, Carter RG, Rocci E, et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J Allergy Clin Immunol. 2015; 136: 737-746.
- Detwiller KY, Smith TL, Alt JA, Trune DR, Mace JC, Sautter NB. Differential expression of innate immunity genes in chronic rhinosinusitis. Am J Rhinol Allergy. 2014; 28: 374–377.
- Jardeleza C, Jones D, Baker L, Miljkovic D, Boase S, Tan NC, et al. Gene expression differences in nitric oxide and reactive oxygen species regulation point to an altered innate immune response in chonic rhinosinusitis. Int Forum Allergy Rhinol. 2013; 3: 193–198.
- Van Drunen CM, Mjosberg JM, Segboer CL, Cornet ME, Fokkens WJ. Role of innate immunity in the pathogenesis of chronic rhinosinusitis: progress and new avenues. Curr Allergy Asthma Rep. 2012; 12: 120–126.
- Yazdani N, Amoli MM, Naraghi M, Mersaghian A, Firouzi F, Sayyahpour F, et al. Association between the functional polymorphism C-159T in the CD14 promoter gene and nasal polyposis: Potential role in asthma. J Investig Allergol Clin Immunol. 2012; 22: 406–411.
- Zhang XH, Zhang YN, Bin LH, Hu CY, Wang N, Cao PP, et al. Overexpression of miR-125b, a novel regulator of innate immunity, in eosinophilic chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2012; 185: 140–151.
- Boita M, Garzaro M, Raimondo L, Riva G, Mazibrada J, Pecorari G, et al. Eosinophilic inflammation of chronic rhinosinusitis with nasal polyps is related to OX40 ligand expression. Innate Immun. 2014; 21: 167–174.
- Jin J, Rha KS, Kim DW, Kim YM. IL-17C expression in nasal epithelial cells of chronic rhinosinusitis with nasal polyposis. 2013; 271: 1097-1105.
- Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy Eur J Allergy Clin Immunol. 2014; 60: 1154–1161.
- Schicht M, Knipping S, Hirt R, Beileke S, Sel S, Paulsen F, et al. Detection of surfactant proteins A, B, C, and D in human nasal mucosa and their regulation in chronic rhinosinusitis with polyps. Am J Rhinol Allergy. 2013; 27: 24–29.
- Shaw J L, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013; 188: 432–439.
- Ba L, Du J, Liu F, Yang F, Han M, Liu S, et al. Distinct Inflammatory Profiles in Atopic and Nonatopic Patients with Chronic Rhinosinustis Accompanied by Nasal Polyps in Western China. Allergy Asthma Immunol Res. 2015; 7: 346–358.
- Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015; 64: 121–130.
- Bae JS, Pasaje CF, Park BL, Cheong HS, Kim JH, Uh ST, et al. Genetic association analysis of CIITA variations with nasal polyp pathogenesis in asthmatic patients. Mol Med Rep. 2013; 7: 927-934.
- Kosugi EM, De Camargo-Kosugi CM, Weckx LL, Guerreiro-da-Silva ID, Gregorio LC. Interleukin-6 -174 G/C promoter polymorphism and nasal polyposis. Rhinology. 2009; 47: 400–404.
- Benito Pescador D, Isidoro-Garcia M, Garcia-Solaesa V, Pascual de Pedro M, Sanz C, Hernandez-Hernandez L, et al. Genetic association study in nasal polyposis. J Investig Allergol Clin Immunol. 2012; 22: 331–340.
- Mfuna-Endam L, Zhang Y, Desrosiers MY. Genetics of rhinosinusitis. Curr Allergy Asthma Rep. 2011; 11: 236–246.
- Zhang Y, Wang X, Zhang W, Han D, Zhang L, Bachert C. Polymorphisms in thymic stromal lymphopoietin gene demonstrate a gender and nasal polyposis-dependent association with chronic rhinosinusitis. Hum Immunol. 2013; 74: 241–248.
- Park M, Suh DS, Lee K, Bae J. Positive cross talk between FOXL2 and antimullerian hormone regulates ovarian reserve. Fertil Steril. 2014; 102: 847-855.
- Mrowicka M, Zielinska-Blizniewska H, Milonski J, Majsterek I, Olszewski J. Association of IL1β and IL4 gene polymorphisms with nasal polyps in a Polish population. Mol Biol Rep. 2014; 41: 4653–4658.
- Davidsson A, Anderson T, Hellquist HB. Apoptosis and phagocytosis of tissue-dwelling eosinophils in sinonasal polyps. Laryngoscope. 2000; 110: 111–116.
- Fang SY, Yang BC. Overexpression of Fas-ligand in human nasal polyps. Ann Otol Rhinol Laryngol. 2000; 109: 267–270.
- Garavello W, Vigano P, Romagnoli M, et al. Expression of cell cycle regulatory proteins and analysis of apoptosis in normal nasal mucosa and in nasal polyps. Am J Rhinol. 2014; 19: 549–553.
- Ingle RR, Setzen G, Koltai PJ, Monte D, Pastore J, Jennings TA. p53 protein expression in benign lesions of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 1997; 123: 297–300.
- Kupper DS, Valera FCP, Malinsky R, et al. Expression of apoptosis mediators p53 and caspase 3, 7, and 9 in chronic rhinosinusitis with nasal polyposis. Am J Rhinol Allergy. 2014; 28: 187–191.
- Lavezzi A, Mantovani M, Cazzullo A, Turconi P, Matturri L. p53 over-expression and its correlation with PCNA index in nasal polyps. Rhinology. 1999; 37: 160–163.
- Chalastras T, Athanassiadou P, Patsouris E, Eleftheriadou A, Kandiloros D, Papaxoinis K, et al. Differential rates of proliferation and apoptosis in nasal polyps correspond to alterations in DNA spatial distribution and nuclear polarization as observed by confocal microscopy. Eur Arch Oto-Rhino-Laryngology. 2010; 267: 1075–1080.
- Wu CC, Lee TJ, Chang PH, Tsai CN, Lee YS, Fu CH, et al. Similar cellular proliferation activities in nasal polyps and adjacent inferior turbinate. Am J Otolaryngol. 2012; 33: 14–19.
- Li CW, Cheung W, Lin ZB, Li TY, Lim JT, Wang DY. Oral steroids enhance epithelial repair in nasal polyposis via. upregulation of the AP-1 gene network. Thorax. 2009; 64: 306–312.
- Hirahara K, Vahedi G, Ghoreschi K, Yang XP, Nakayamada S, Kanno Y, et al. Helper T-cell differentiation and plasticity: insights from epigenetics. Immunology. 2011; 134: 235-245.
- Pezato R, Perez-Novo CA, Holtappels G, De Ruyck N, Van Crombruggen K, De Vos G, et al. The expression of dendritic cell subsets in severe chronic rhinosinusitis with nasal polyps is altered. Immunobiology 2014; 19: 729-736.
- Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61: 1280-1289.
- Huang L, Baban B, Johnson BA, Mellor AL. Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int Rev Immunol. 2010; 29: 133–155.
- Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011; 128: 728-732.
- Luong A, Davis LS, Marple BF. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy. 2009; 23: 281-287.
- Hammad H, Lambrecht BN. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy 2011; 66: 579-587.
- Schleimer RP, Lane AP, Kim J. Innate and acquired immunity and epithelial cell function in chronic rhinosinusitis. Clin Allergy Immunol. 2007; 20: 51-78.
- Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122: 961-968.
- Wang ET, Zheng Y, Liu PF, Guo LJ. Eosinophilic chronic rhinosinusitis in East Asians. World J Clin Cases. 2014; 2: 873-882.
- Derycke L, Eyerich S, Van Crombruggen K, Perez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014; 9: 97581.
- Bachert C, Zhang N, Holtappels G, De Lobel L, Van Cauwenberge P, Liu S, Lin P, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbidsthma. J Allergy Clin Immunol. 2010; 126: 962–968.
- Pezato R, Swierczynska-Krepa M, Nizankowska-Mogilnicka E, Holtappels G, De Ruyck N, Sanak M, et al. Systemic expression of inflammatory mediators in patients with chronic rhinosinusitis and nasal polyps with and without Aspirin Exacerbated Respiratory Disease. Cytokine. 2016; 77: 157-167.
- Cho KS, Kim YW, Kang MJ, Park HY, Hong SL, Roh HJ. Immunomodulatory Effect of Mesenchymal Stem Cells on T Lymphocyte and Cytokine Expression in Nasal Polyps. Otolaryngol Head Neck Surg. 2014; 150: 1062-1070.
- Cho J S, Park J H, Kang J H, Kim S E, Park I H, Lee H M. Isolation and characterization of multipotent mesenchymal stem cells in nasal polyps. Exp Biol Med. 2015; 240: 185-193.
- Li X, Meng J, Qiao X, Liu Y, Liu F, Zhang N, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol. 2010; 125: 1061–1068
- Van Bruaene N, Derycke L, Perez-Novo C, Gevaert P, Holtappels G, De Ruyck N, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009; 124: 253-259.
- Lee YM, Kim SS, Kim HA, Suh YJ, Lee SK, Nahm DH, et al. Eosinophil inflammation of nasal polyp tissue: relationships with matrix metalloproteinases, tissue inhibitor of metalloproteinase-1, and transforming growth factor-beta1. J Korean Med Sci. 2003; 18: 97–102.
- Liu Z, Gao Q, Zhang S, You X, Cui Y. Expression of tenascin and fibronectin in nasal polyps. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2002; 37: 173-176.
- Hatziri A, Vynios DH, Panogeorgou T, Bouga H, Triantaphyllidou IE, Naxakis SS, et al. Presence of hyaluronidase isoforms in nasal polyps. Eur Rev Med Pharmacol Sci. 2013; 17: 247-252.
- Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013; 132: 584-592.
- Balsalobre L, Pezato R, Perez-Novo C, Alves MT, Santos RP, Bachert C, et al. Epithelium and stroma from nasal polyp mucosa exhibits inverse expression of TGF-β1 as compared with healthy nasal mucosa. J Otolaryngol Head Neck Surg. 2013; 42: 29.
- Figueiredo CR, Santos RP, Silva ID, Weckx LL. Microarray cDNA to identify inflammatory genes in nasal polyposis. Am J Rhinol. 2007; 21: 231–235.
- Sejima T, Holtappels G, Bachert C. The expression of fibrinolytic components in chronic paranasal sinus disease. Am J Rhinol Allergy. 2011; 25: 1–6.
- De Borja Callejas F, Picado C, Martinez-Anton A, Alobid I, Pujols L, Valero A, et al. Differential expression of remodeling markers by tissue structure in nasal polyposis. Am J Rhinol Allergy. 2013; 27: 69-74.
- Pezato R, Voegels RL. Why do we not find polyps in the lungs? Bronchial mucosa as a model in the treatment of polyposis. Med Hypotheses. 2012; 78: 468-470.
- Pezato R, Voegels RL, Pinto Bezerra TF, Perez-Novo C, Stamm AC, Gregorio LC. Mechanical disfunction in the mucosal oedema formation of patients with nasal polyps. Rhinology. 2014; 52: 162-166.
- Da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & Growth Factor Reviews. 2009; 20: 419-427.
- Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annual Review of Medicine. 2008; 59: 311-325.
- Ninichuk V, Gross O, Segerer S, Hoffmann R, Radomska E, Buchstaller A, et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006; 70: 121-129.
- Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009; 27: 3063-3073.
- Donizetti-Oliveira C, Semedo P, Burgos-Silva M, Cenedeze MA, Malheiros DM, Reis MA, et al. Adipose tissue-derived stem cell treatment prevents renal disease progression. Cell Transplant. 2012; 21: 1727-1741.
- Li W, Jiang H, Feng JM. Isogenic mesenchymal stem cells transplantation improves a rat model of chronic aristolochic acid nephropathy via. upregulation of hepatic growth factor and downregulation of transforming growth factor β1. Mol Cell Biochem. 2012; 368: 137-145.
- Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract. 2012; 98: 465-473.
- Qi Y, Jiang D, Sindrilaru A, Stegemann A, Schatz S, Treiber N, et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J Invest Dermatol. 2014; 134: 526-537.
- Kim MD, Kim SS, Cha HY, Jang SH, Chang DY, Kim W, et al. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp Mol Med. 2014; 46: 110.
- Polacek M, Bruun JA, Elvenes J, Figenschau Y, Martinez I. The secretory profiles of cultured human articular chondrocytes and mesenchymal stem cells: implications for autologous cell transplantation strategies. Cell Transplant. 2011; 20: 1381-1393.
- Mannello F, Tonti GA, Bagnara GP, Papa S. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem Cells. 2006; 24: 475-481.
- Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via. distinct cytokine and protease expression mechanisms. Angiogenesis. 2011; 14: 47-59.
- Shu T, Zeng B, Ren X, Li Y. HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue Cell. 2012; 42: 217-222.
- Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009; 27: 2734-2743.