
Research Article
Austin Immunol. 2016; 1(2): 1007.
Validation of Two New Immunoassays for Sensitive Detection of a Broad Range of Shiga Toxins
Kong Q¹, Patfield S², Skinner C², Stanker LH², Gehring AG³, Fratamico PM³, Rubio F4, Qi W¹* and He X²*
¹Shanghai Shuneng Irradiation Technology Co., Ltd, Shanghai Academy of Agricultural Sciences, China
²Western Regional Research Center, U.S. Department of Agriculture, Agricultural Research Service, USA
³Eastern Regional Research Center, U.S. Department of Agriculture, Agricultural Research Service, USA
4Abraxis, USA
*Corresponding author: Xiaohua He, Western Regional Research Center, U.S. Department of Agriculture, Agricultural Research Service, 800 Buchanan St., Albany, CA94710, USA
Qi Wenyuan, Shanghai Shuneng Irradiation Technology Co., Ltd, Shanghai Academy of Agricultural Sciences, 1000 Jinqi Rd., Shanghai 20143, China
Received: July 13, 2016; Accepted: August 26, 2016; Published: August 30, 2016
Abstract
Shiga Toxin (Stx) is one of the major virulence factors produced by Shiga Toxin-producing E. coli (STEC) that cause severe human intestinal diseases. Although a few commercial assays for Stxs are available, they only detect a subset of Stxs. In this study, two new immunoassays, Abraxis Stx1 and Stx2, were evaluated and compared with the widely used Premier EHEC kit using the same set of standards developed in our laboratory. The new assays were demonstrated to be highly reliable and capable of detecting all 10 subtypes of Stxs and have a limit of detection for Stx1a and Stx2a down to 25 pg/mL, a 20- fold improvement over the Premier EHEC. When applied to forty-nine bacterial isolates collected from clinic, environmental and fresh produce samples, the new assays identified all but one stx2b-producing STEC strains, while the Premier EHEC ELISA missed two stx2e- and one stx2g-STEC stains. Furthermore, the new assays were also able to identify STEC strains using single colonies on agar plates without lengthy enrichment in liquid medium. The broad crossreactivity, robustness and high reproducibility and sensitivity of the new assays will be useful in reducing product recalls due to failures of detecting rare Stxs.
Keywords: Abraxis Stx ELISA; Microplate assay; Premier EHEC; Shiga toxin-producing E. coli; Stx1 subtypes; Stx2 subtypes
Abbreviations
E. coli: Escherichia coli; ELISA: Enzyme-Linked Immunosorbent Assay; HUS: Hemolytic Uremic Syndrome; Stx: Shiga Toxin; STEC: Shiga Toxin-producing Escherichia coli
Introduction
Disease outbreaks caused by Shiga Toxin (Stx)-producing Escherichia coli (STEC) have occurred with increasing frequency [1]. Although most STEC-caused illness usually resolves itself, Hemolytic Uremic Syndrome (HUS) can occur in susceptible individuals, particularly in-children and the elderly, resulting in chronic and irreversible renal dysfunction or even death [2]. Currently, there is no effective therapeutic method available for HUS besides supportive care. The advent of better diagnostic methods for STEC in different matrices is crucial to prevent susceptible patients from developing HUS. Conventionally, detection of STEC was based on the unique sorbitol negative fermentation property of E. coli O157:H7, therefore, this organism was the most commonly recognized serotype associated with outbreaks [3]. However, it has become clear now that numerous non-O157 STEC serogroups, including the Big Six (O26, O45, O103, O111, O121, and O145) and serotype O104:H4 also can cause serious human illness and outbreaks [4,5]. Therefore, better diagnostic strategies are needed. Stx is one of the most important virulence factors of STECs and the production of Stxs is the common trait of all STEC strains, therefore, a non-culture method relying on the production of Stx as a marker would be a good alternative approach to the culture assays. Activity-based assays such as Vero cell and mouse bioassays have played an important part in the detection of Stxs, but these assays are laborious and non-specific, and a subsequent assay is required to confirm the presence of the Stx. Immunoassays have been popular because they are not only sensitive and specific, but also easy, robust, and all reagents and equipment needed are available in most laboratories. There are two types of Stx produced by E. coli strains, Stx1 and Stx2. Three subtypes of Stx1 (Stx1a, Stx1c, and Stx1d) and seven subtypes of Stx2 (Stx2a to Stx2g) have been isolated according to a recent sequence-based classification [6]. Although these toxins are similar to each other structurally and functionally [7,8], their broad genetic variations present a challenge for the development of a universal immunoassay that detects all subtypes of Stxs.
Currently, there are four FDA-approved immunoassays for STEC detection available in the United States [9], two in microplate format: the Premier EHEC (Meridian Diagnostics, Cincinnati, Ohio) and the ProSpecT Shiga Toxin E. coli Microplate Assay (Remel, Lenexa, Kansas) and two in lateral flow device format: the Immunocard STAT! EHEC (Meridian Diagnostics) and the Duopath Verotoxins Gold labeled immunosorbent Assay (Merck, Germany). Studies on sensitivities and specificities of these commercial assays for the most common subtypes of Stxs, Stx1a and Stx2a, have been reported [10- 15] but no data could be found for the ability of these assays to detect the less common subtypes of Stxs, such as Stx1d and Stx2b, 2e, and 2f. Also, direct comparisons among different assays have not been done.
In this study, we provided first-hand evidence on the sensitivity and specificity of two new commercial Stx assays in detecting all subtypes of Stxs (3 Stx1s and 7 Stx2s) and compared their performance with the Premier EHEC kit using the same set of standards and fresh cultures of 49 bacterial collections.
Materials and Methods
Bacterial strains
Forty-nine bacterial strains with different stx genotypes were tested in this study and some of their characteristics are indicated in (Table 3). These isolates came from the bacterial strain collection housed in the Produce Safety and Microbiology Research Unit at USDA, ARS, Western Regional Research Center and from the Molecular Characterization of Foodborne Pathogens Research Unit at USDA, ARS, Eastern Regional Research Center. Stock bacterial strains were maintained in 20% glycerol and frozen at -80°C. Fresh bacterial cultures were produced by inoculating frozen stock cultures onto Tryptic Soy Agar (TSA) plates and incubating the plates overnight at 37°C.
Strain
Origin
Serotype
stx gene
cfu/mL x 108
Abraxis-Stx1
Abraxis-Stx2
PremierEHEC
RM1239
Human
O157:H7
stx2a
3.45
-
++
++
RM1913
Human
O157:H7
stx2a
2.93
-
+++
+++
RM2367
Human
O157:H7
stx1a, stx2a
2.28
++++
+++
+++
RM5856
Human
O121:H19
stx2a
2.25
-
+++
+++
RM6649
Human
O157:H7
stx1a, stx2a
2.95
++++
+++
+++
RM6848
Lettuce
O121
stx2a
2.53
-
++++
+++
RM7005
Human
O118:H12
stx2b
nd
-
-
+
RM7007
Feral Pig
O128:H2
stx2f
nd
-
+++
++
RM7110
Pig
O139:NM
stx2e
nd
-
+
-
RM7370
Water
O111
stx1a, stx2a
3.03
++++
++
+++
RM7375
Human
O26
stx1a
4.67
++++
-
+++
RM7543
Human
O157:H7
stx1a, stx2a
2.45
++++
++
+++
RM7783
Crow
O113
stx2a
2.70
-
++
+
RM7788
Water
O113
stx2a
3.30
-
+++
++
RM7927
Water
O26
stx1a
3.05
++++
-
+++
RM7958
Cow feces
O113
stx1a, stx2d
2.87
++++
+++
++++
RM7988
nd
nd
stx2e
nd
-
+
-
RM8013
Cow
nd
stx2d
nd
-
+++
+++
RM8082
Cow feces
O121
stx1d
3.17
+
-
++
RM8352
Sediment
O121
stx2a
2.37
-
++++
+++
RM8385
nd
O103
stx1a
4.00
+++
-
+++
RM8426
Water
O26
stx1a
3.67
++++
-
+++
RM8876
Water
O145
stx1a
3.95
++++
-
+++
RM9306
Cow feces
O145
stx1a
3.45
++++
-
+++
RM9322
Water
O111
stx1a
3.77
++++
-
++++
RM9413
Cow feces
O45
stx1a
4.55
+++
-
+++
RM9872
Cow feces
O145
stx2a
3.77
-
++++
+++
RM9882
Cow feces
O103
stx1a
3.57
++++
-
++++
RM9907
Feral Pig
O111
stx1a
3.40
++++
-
+++
RM9917
Feral Pig
O145
stx1a
3.30
++++
-
+++
RM9975
Crow
O111
stx1a
3.45
++++
-
+++
RM10058
Crow bird
O157:H7
stx2c
nd
-
++++
++
RM10061
Feral Pig
O103
stx1a
3.45
++++
-
+++
RM10408
Crow
O103
stx1a
2.45
++++
-
++++
RM10466
Cow feces
O113
stx2a
3.00
-
+++
+++
RM10468
Watershed
nd
stx2g
nd
-
++
-
RM10817
Cow feces
O26
stx1a
3.22
++++
-
+++
RM10940
Cow feces
O113
stx2a
2.87
-
+++
+++
RM12238
Human
O145
stx2a
2.92
-
++
++
RM12788
Human
O111
stx1a, stx2a
3.25
++++
++++
++++
RM13149
nd
nd
stx1d
nd
+
-
+
RM13504
nd
O121
stx2a
2.77
-
++++
+++
RM13506
Human
O45
stx1a
3.12
++++
-
++++
RM13508
Human
O103
stx1a
4.22
++++
-
++++
RM13752
Cow feces
O45
stx1a
3.87
+++
-
++
CC3
nd
O128ac:H2
stx2f
nd
-
+++
++
II9
nd
O41:H26
stx1d
nd
+
-
+
ATCC25922
Clinic
O6
-
3.80
-
-
-
RM7103
nd
O45
-
nd
-
-
-
(-) Controlb
-
-
-
-
(+) Controlb
+
+
+
+
aAbraxis Stx1 kit: + indicates ELISA signal to noise ratio (s/n) > 2 or OD450 > 0.16. OD450 between 0.16 and 1, +; OD450 between 1 and 2, ++; OD450 between 2 and 3, +++; OD450 > 3, ++++
Abraxis Stx2 kit and Premier EHEC kit: + indicates s/n > 2 or OD450 > 0.26. OD450 between 0.26 and 1, +; OD450 between 1 and 2, ++; OD450 between 2 and 3, +++; OD450 > 3, ++++
nd: not determined.
Table 3: ELISA Detection of Stx produced by enriched broth cultures of STECa.
Preparation of bacterial samples for ELISA
Axenic broth cultures for ELISA were prepared as described previously with minor modifications [16]. Briefly, a fresh colony (~ 1 mm in diameter) of each bacterial strain was inoculated in a Falcon tube containing 1 mL Tryptic Soy Broth (TSB) with 50 ng/mL mitomycin C and 10 g/L casamino acid, and incubated for 2 h at 37°C with shaking. Bacterial cultures were then diluted to a desired CFU/ mL (2.25 x 108 to 4.67 x 108) and then treated with an equal volume of phosphate Bacterial Protein Extraction Reagent (B-PER, Pierce Biotechnology, Rockford, IL) for 1 h at 37°C. Following centrifugation at 13,000 x g for 10 min at 4°C, bacterial culture supernatants were collected for use in ELISAs.
For colony ELISA, colonies (~ 1 mm in diameter) from TSA plates were picked using a pipette tip and re-suspended in wells of a clean microplate containing 100 μL TSB with 50 ng/mL mitomycin C and 100 μL B-PER. After incubation at 37°C for 1 h, 100 μL of clear supernatant was removed from each well after centrifugation and added to an ELISA plate pre-coated with capture antibodies for Stx analysis.
ELISAs
Toxoids of Stx1a, 1c, 1d and Stx2a through 2g were prepared as described [17] and used as standards in ELISAs. The Abraxis Stx1 and Stx2 ELISA kits were obtained from Abraxis LLC (Product #542000 and #542010, Warminster, PA) and the Premier EHEC assay was purchased from Meridian Bioscience Inc. (Catalogue #608096, Cincinnati, OH). ELISA reagents and buffers were prepared according to manufacturers’ instructions. Test samples (100 μL/well) were added to the ELISA plate, and the assay was performed according to kit instructions. Absorbance values for samples were read on a Victor 3 plate reader (Perkin Elmer, Waltham, MA) after addition of the stop solution. Values represent the mean from three experiments. A semiquantitative analysis was performed based on the ELISA absorbance value at wavelength 450 nm. The LOD was calculated by extrapolating ng/mL of Stxs from the average background ELISA reading plus three standard deviations of the background.
Inter- and intra-assay coefficient of variability
In order to measure the precision, or repeatability of the new Abraxis ELISA kits, the inter- and intra-assay Coefficient of Variability (CV) was examined. In this study, intra-assay precision was determined for each of 4 concentrations of Stx1 and Stx2 standards from 5 assay runs per day, each run includes 5 replicates of each concentration. The mean readings of ELISA OD450 at each concentration were calculated from 5 run and 5 replicates per run (total 25 replicates). The % CV for each concentration is calculated by dividing the Standard Deviation (SD) by the mean, and multiplying by 100. The average intra-assay CV was obtained by averaging individual CVs from each day, over 5 non-consecutive days. The inter-assay precision was determined by dividing the SD of 5 day means by mean of 5 day means multiplying by 100.
Robustness of the assay
The robustness of a procedure is a measure of its capacity to remain unaffected by small variations during testing. All experiments were performed following manufacturer’s instruction. Variations assessed for their effect on the assay value was: assay temperature, sample (Stx1 and Stx2), detecting antibody and enzyme conjugate incubation time. Three replicates per day and two separate days were analyzed for each conditions tested. Mean OD450 readings for positive Stx controls were analyzed.
Statistical analyses
For all comparative analyses between conditions for robustness, means, standard deviations, and CVs were collected for assay positive controls and test samples, and compared to the standard assay procedure using Analysis of Variance (ANOVA). A p-value = 0.05 was used to determine statistical difference. Test conditions were considered equivalent unless they were both statistically significant different (p = 0.05) and % CV = 15%.
Results and Discussion
Precision of the assay
To evaluate the precision of the new Abraxis Stx detection kits, it is necessary to measure the repeatability (intra-day) and the reproducibility (inter-day) of these assays. The intra- and interassay precision (% CV) was determined by analyzing serial dilutions of Stx1 (Table 1) and Stx2 (Table 2) spiked in Phosphate Buffered Saline (PBS). The average intra-assay precision expressed as % CV for Stx1 at concentrations of 0, 0.1, 0.25, 0.5 ng/mL were 8.13, 5.39, 4.64 and 4.86, respectively, for Stx2 at concentrations of 0, 0.25, 0.5, and 1 ng/mL were 3.58, 2.91, 3.1, and 3.49, respectively. The inter-assay precision (% CV) for Stx1 at concentrations of 0, 0.1, 0.25, and 0.5 ng/ mL ranged from 4.91 to 8.28, for Stx2 at concentrations of 0, 0.25, 0.5, and 1 ng/mL ranged from 5.54 to 7.63. In general, inter-assay % CVs of less than 15 and intra-assay % CV less than 10 are accepted. These data indicate that the Abraxis Stx assays are highly reproducible.
Intra-day precision
Stx1 standard (ng/mL)
Replicates
Mean reading of OD450 nm
Standard deviation
% CV
Day 1
0
25
0.133
0.008
6.28
0.1
25
0.214
0.010
4.74
0.25
25
0.348
0.012
3.38
0.5
25
0.589
0.044
7.54
Day 2
0
25
0.128
0.014
10.54
0.1
25
0.203
0.011
5.48
0.25
25
0.326
0.018
5.54
0.5
25
0.527
0.025
4.83
Day 3
0
25
0.121
0.012
10.17
0.1
25
0.189
0.014
7.32
0.25
25
0.300
0.012
4.14
0.5
25
0.505
0.016
3.22
Day 4
0
25
0.137
0.007
5.41
0.1
25
0.211
0.008
3.59
0.25
25
0.334
0.019
5.63
0.5
25
0.576
0.024
4.10
Day 5
0
25
0.151
0.012
8.25
0.1
25
0.209
0.012
5.83
0.25
25
0.323
0.015
4.50
0.5
25
0.543
0.025
4.61
Inter-day
0
125
0.134
0.011
8.28
precision
0.1
125
0.205
0.010
4.91
0.25
125
0.326
0.018
5.45
0.5
125
0.548
0.034
6.27
Table 1: Assessment of intra- and inter-assay precision for the Abraxis Stx1 detection kit.
Intra-day precision
Stx2 standard (ng/mL)
Replicates
Mean reading of OD 450 nm
Standard deviation
% CV
Day 1
0
25
0.256
0.011
4.31
0.25
25
0.407
0.012
2.94
0.5
25
0.567
0.018
3.14
1
25
0.918
0.029
3.11
Day 2
0
25
0.254
0.005
2.05
0.25
25
0.385
0.012
3.06
0.5
25
0.529
0.013
2.50
1
25
0.838
0.027
3.18
Day 3
0
25
0.264
0.006
2.21
0.25
25
0.372
0.008
2.25
0.5
25
0.492
0.010
2.12
1
25
0.761
0.024
3.19
Day 4
0
25
0.298
0.017
5.73
0.25
25
0.421
0.014
3.38
0.5
25
0.548
0.026
4.65
1
25
0.841
0.038
4.48
Inter-day
0
100
0.268
0.020
7.63
precision
0.25
100
0.396
0.022
5.54
0.5
100
0.534
0.032
6.00
1
100
0.840
0.064
7.63
Table 2: Assessment of intra- and inter-assay precision for the Abraxis Stx2 detection kit.
Robustness of the assay
Incubation times were tested for three steps of the assay’s procedure: Stx (Stx1 and Stx2), detecting antibody, and enzyme conjugate incubation times. There was no statistical difference between the standard Stx incubation time of 1 hour and the varying conditions (55 min or 65 min). The CVs between different incubation times ranged from 10 to 13%. Change in detecting antibody and enzyme conjugation incubation time from 30 min to 27 min or to 33 min resulted in % CV from 3.4 to 8.9%, and no statistical difference was found. Change in Stx1 and Stx2 assay temperature from 25C to 28C resulted in % CV from 2.19 to 5.36% and no discernible difference was found.
Detection of different subtypes of Stx1 and Stx2
Both Abraxis Stx1 and Stx2 ELISAs are performed in microplate format, similar to the Premier EHEC test. To reliably evaluate the sensitivity and specificity of these assays for different subtypes of Stx1 and Stx2, one of the most important things to consider is the standards. Similar studies have been reported [10,11,14] for existing commercial assays, but no conclusions could be drawn because the standards used by different investigators were not the same, crude Stx samples, such as bacterial culture supernatants or cell lysates were often used, so the actual sensitivity of the assay was not known. In this study, we intended to make a direct comparison between kits made by the companies Abraxis LLC (Abraxis Stx1 ELISA and Abraxis Stx2 ELISA) and Meridian Bioscience, Inc. (Premier EHEC) using the same set of standards. Because commercial standards for most subtypes of Stxs are not available, and purifying Stxs from pathogenic bacterial strains is difficult due to the relatively low amount of toxins expressed, especially for some subtypes such as Stx2b, 2e and 2f [13], toxin standards were made through recombinant DNA approaches. However, considering public biosecurity concerns on generating biologically active recombinant toxins, instead we used non-toxic recombinant toxoids, Stx1aE167Q, Stx1cE167Q, Stx1dE167Q, Stx2aE167Q, Stx2bE167Q, Stx2cE167Q, Stx2dE167Q, Stx2eE167Q, Stx2fE167Q, and Stx2gE167Q, prepared previously [17], as alternative standards for Stx1a, 1c, 1d, Stx2a, 2b, 2c, 2d, 2e, 2f, and 2g, respectively. These toxoids were created by converting the conserved glutamic acid at position 167 of their native toxins to glutamine [18]. The toxicities of these toxoids were destroyed but their structure remained unchanged [19]. Figure 1 demonstrates that the Abraxis Stx1 and Stx2 ELISAs were able to detect all 10 subtypes of Stx1 (Figure 1a) and Stx2 (Figure 1b) toxoids at 10 ng/mL, but their sensitivities to different subtypes varied, significantly, based on ELISA signals (absorbance at 450 nm). The ELISAs were the most sensitive to Stx1a and Stx2a, and least sensitive to Stx1d and Stx2b, respectively. For most subtypes, the Abraxis ELISAs were more sensitive than the Premier EHEC kit, but for the Stx1d, Stx2b and Stx2d, the Premier EHEC gave greater signals than did the Abraxis ELISAs (p< 0.01). However, the Premier EHEC failed to detect the Stx2g at 10 ng/mL (Figure 1b). As a commercial kit, the ability to detect all subtypes of Stxs is critical. Although Stx2a, Stx2c, and Stx2d have been more frequently linked to the development of HUS [20,21], other subtypes are also associated with human illness. For example, Stx2e-producing strains normally cause edema disease in pigs and do not represent a particular threat for humans [22], however, STEC carrying the stx2e gene have been isolated from human cases with mild diarrhea [23,24] and from two patients with HUS [25,26]. Assays with limited sensitivity and cross-reactivity to all subtypes of Stxs could give falsenegative conclusions, resulting in serious impact on human health.
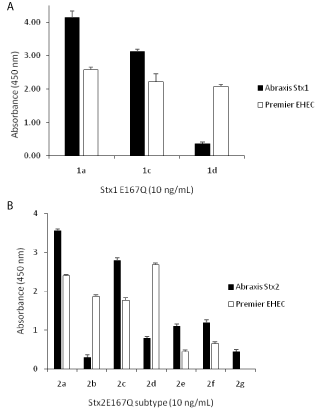
Figure 1: Cross-reactivity of Abraxis and Premier EHEC ELISAs to different
subtypes of Stxs.
Toxoids of Stx1 (Figure 1a) and Stx2 (Figure 1b) were used as standards and
ELISAs were performed following manufacturers’ instruction. Data shown
represent the mean plus SD. Three individual experiments were performed.
Detection limits for Stx1a and Stx2a toxoids
One of the differences between the Abraxis and Meridian ELISAs is that the Abraxis ELISAs are able to differentiate Stx1 from Stx2, but the Premier EHEC assay does not. To compare their sensitivities for the common Stx subtypes, Stx1a and Stx2a, serial dilutions (0, 0.025, 0.05, 0.5, 1, 5, 10 ng/mL) of the corresponding toxoids were prepared in PBS, and ELISAs were performed following the manufacturers’ instructions. Our results showed that there was a linear correlation between the toxin concentration and the ELISA absorbance at 450 nm between the range of 0.025 ng/mL and 10 ng/mL using both ELISA kits, and all correlation coefficients were high (0.99). However, the calibration curves obtained using the Abraxis Stx1 and Stx2 ELISAs had steeper slopes than those obtained using the Premier EHEC ELISA (p< 0.01, Figure 2). The Limit of Detection (LOD) for Stx1a and Stx2a toxoids was 25 pg/mL using the Abraxis Stx1 and Stx2 ELISAs and 500 pg/mL using the Premier EHEC ELISA, suggesting that the new Abraxis ELISAs are more sensitive for Stx1a and Stx2a than the Premier EHEC ELISA.
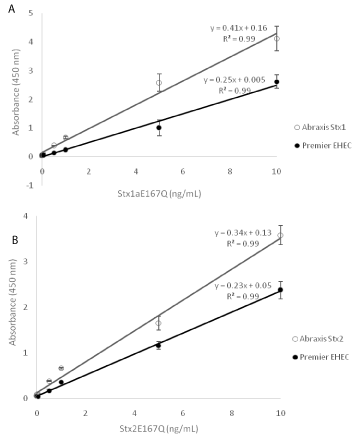
Figure 2: Sensitivity of Abraxis and Premier EHEC ELISAs.
Serial dilutions of Stx1a (Figure 2a) and Stx2a (Figure 2b) toxoids (0, 0.025,
0.05, 0.5, 1.0, 5.0, 10 ng/mL) were prepared in PBS buffer and used as
standards. ELISAs were performed following manufacturers’ instruction.
Data shown represent the mean plus SD. Three individual experiments were
performed.
Detection of Stx generated from STEC enrichment cultures
To compare the performance of three commercial assays for detection of native Stxs produced by STEC strains, single colonies from 49 bacterial strains representing 13 serotypes and 4 un-typed bacterial strains with different stx genotypes were inoculated into TSB containing mitomycin C and incubated for 2 h at 37°C (to induce Stx production, and referred to as enrichment cultures) and then treated with B-PER to release cell-associated Stx. This step is important particularly for Stx1 detection because Stx1 is known to be closely associated with cells [27]. In Table 3, ELISA results obtained from the Abraxis Stx1 and Stx2 ELISAs and the Premier EHEC ELISA are shown. The semi-quantitative results were obtained based on the ELISA absorbance values at wavelength 450 nm. Both Abraxis Stx1 and Premier EHEC ELISAs were able to identify all STEC strains carrying the stx1 genes. The Abraxis Stx2 ELISA was able to identify all Stx2-producing STEC strains, except for the strain, RM7005, which carries a stx2b gene. The Premier EHEC ELISA successfully detected Stx2 produced by most of the STEC strains, but failed to identify two STEC strains that carried stx2e, RM7110, RM7988, and one stx2g-STEC strain, RM10468. These results are in agreement with our observations found with the pure toxoids, which indicates the low reactivity of the Abraxis Stx2 ELISA for detecting Stx2b and the Premier EHEC for detecting Stx2e and Stx2g. It is possible that the antibodies incorporated in these kits had low cross-reactivity to the corresponding toxins or toxin levels expressed by these strains were extremely low (below the LOD), resulting in detection failure. The majority strains tested in this study were Stx1a and/or Stx2a producers because they are the most common subtypes produced by STECs and were available in our laboratory. More STEC strains that produce rare subtypes of Stxs, such as Stx2b, 2e, 2f, and 2g would give us better estimation on the capacity of these new ELISA kits for detection of Stxs.
To investigate the potential of the new commercial ELISAs for identification of -STEC strains- without lengthy liquid enrichment steps, single colonies of bacterial strains (1 mm in diameter) collected from TSA plates were directly examined for the production of Stxs. Our results indicate that all STEC strains were identifiable relying on the Stx markers, and no false positive results were found for non- STEC strains (Table 4).
Strain
stx gene
OD450
SD
Stx1 ELISA result
OD450
SD
Stx2 ELISA result
2367
stx1a, stx2a
2.78
0.05
+
1.20
0.07
+
6649
stx1a, stx2a
2.61
0.11
+
0.64
0.09
+
7370
stx1a, stx2a
2.68
0.14
+
1.17
0.12
+
7543
stx1a, stx2a
2.27
0.41
+
0.61
0.02
+
9872
stx2a
0.34
0.01
-
1.39
0.76
+
12788
stx1a, stx2a
2.77
0.07
+
0.43
0.01
+
7103
-
0.36
0.02
-
0.14
0.03
-
25922
-
0.35
0.01
-
0.16
0.00
-
Note: When OD450 value > negative control strain OD value plus 3 times SD, the strain is considered as Stx positive.
Table 4: Rapid detection of Stxs by colony ELISA using Abraxis Stx1 and Stx2 kits.
Conclusion
The new Abraxis Stx1 and Stx2 ELISA kits were evaluated for their repeatability and reproducibility. They were also compared with the Premier EHEC kit for their cross-reactivity to different subtypes using the same set of standards and fresh bacterial culture samples. The new kits were demonstrated to be highly reliable and detect all 10 subtypes of Stxs, while the Premier EHEC kit failed to detect Stx2g at 10 ng/mL in phosphate buffer. For most subtypes, the Abraxis kits were more sensitive, however, the Premier EHEC kit performed better for Stx1d, 2b, and 2d, suggesting that these kits are complementary to each other. The sensitivity of the new kits for the Stx1a and Stx2a was a 20-fold improvement over the Premier EHEC kit. When they were used to identify STEC strains based on the production of Stxs, it was shown that both the Premier EHEC and Abraxis kits were capable of detecting Stxs produced by various STEC strains within 2 h following the treatment of the cells with mitomycin C and B-PER. The Abraxis kits identified all STECs except for one stx2b-strain, while the Premier EHEC kit failed to recognize two stx2e- and one stx2g-strain. These results indicate that the new Abraxis Stx kits are excellent additions to the existing commercial Stx detection tools and have great potential for diagnostic purposes in addition to various applications by regulatory agencies, and the food industry alike.
Acknowledgement
This study was supported by USDA-ARS CRIS project 2030- 42000-049-00D. We would like to thank Beatriz Quinones, Michael Cooley, and Anna Bates for providing STEC strains. Mention of brand or firm names does not constitute an endorsement by the USDA over others of a similar nature not mentioned. USDA is an equal opportunity provider and employer.
Author Contributions
Conceived and designed experiments: XH, AG, PF, LS, FR, and WQ. Provided plates, standards, and bacterial strains: FR, XH, and PF. Performed the experiments: QK, SP, and CS. All authors analyzed data and were involved in preparing the manuscript. Wrote the manuscript: XH.
References
- Gyles CL. Shiga toxin-producing Escherichia coli: an overview. J Anim Sci. 2007; 85: 45-62.
- Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991; 13: 60-98.
- Ojeda A, Prado V, Martinez J, Arellano C, Borczyk A, Johnson W, et al. Sorbitol-negative phenotype among enterohemorrhagic Escherichia coli strains of different serotypes and from different sources. J Clin Microbiol. 1995; 33: 2199-2201.
- Muniesa M, Hammerl JA, Hertwig S, Appel B, Brussow H. Shiga toxin-producing Escherichia coli O104:H4: a new challenge for microbiology. Appl Environ Microbiol. 2012; 78: 4065-4073.
- Luna-Gierke RE, Griffin PM, Gould LH, Herman K, Bopp CA, Strockbine N, et al. Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol Infect. 2014; 142: 2270-2280.
- Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol. 2012; 50: 2951-2963.
- Fraser ME, Fujinaga M, Cherney MM, Melton-Celsa AR, Twiddy EM, O'Brien AD, et al. Structure of shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J Biol Chem. 2004; 279: 27511-27517.
- Fraser ME, Chernaia MM, Kozlov YV, James MN. Crystal structures of the holotoxin from Shigella dysenteriae at 2.5 A resolution. Nat Struct Biol. 1994; 1: 59-64.
- Gould LH, Bopp C, Strockbine N, Atkinson R, Baselski V, Body B, et al. Recommendations for diagnosis of shiga toxin--producing Escherichia coli infections by clinical laboratories. MMWR Recomm Rep. 2009; 58: 1-14.
- Vallieres E, Saint-Jean M, Rallu F. Comparison of three different methods for detection of Shiga toxin-producing Escherichia coli in a tertiary pediatric care center. J Clin Microbiol. 2013; 51: 481-486.
- Willford J, Mills K, Goodridge LD. Evaluation of three commercially available enzyme-linked immunosorbent assay kits for detection of Shiga toxin. J Food Prot. 2009; 72: 741-747.
- Manning SD, Madera RT, Schneider W, Dietrich SE, Khalife W, Brown W, et al. Surveillance for Shiga toxin-producing Escherichia coli, Michigan, 2001-2005. Emerg Infect Dis. 2007; 13: 318-321.
- Beutin L, Kruger U, Krause G, Miko A, Martin A, Strauch E. Evaluation of major types of Shiga toxin 2E-producing Escherichia coli bacteria present in food, pigs, and the environment as potential pathogens for humans. Appl Environ Microbiol. 2008; 74: 4806-4816.
- Park CH, Kim HJ, Hixon DL, Bubert A. Evaluation of the duopath verotoxin test for detection of shiga toxins in cultures of human stools. J Clin Microbiol. 2003; 41: 2650-2653.
- Teel LD, Daly JA, Jerris RC, Maul D, Svanas G, O'Brien AD, et al. Rapid detection of Shiga toxin-producing Escherichia coli by optical immunoassay. J Clin Microbiol. 2007; 45: 3377-3380.
- Gehring A, He X, Fratamico P, Lee J, Bagi L, Brewster J, et al. A high-throughput, precipitating colorimetric sandwich ELISA microarray for Shiga toxins. Toxins (Basel). 2014; 6: 1855-1872.
- He X, Kong Q, Patfield S, Skinner C, Rasooly R. A new immunoassay for detecting all subtypes of Shiga toxins produced by Shiga toxin-producing E. coli in ground beef. PLoS One. 2016.
- Hovde CJ, Calderwood SB, Mekalanos JJ, Collier RJ. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc Natl Acad Sci USA. 1988; 85: 2568-2572.
- Gordon VM, Whipp SC, Moon HW, O'Brien AD, Samuel JE. An enzymatic mutant of Shiga-like toxin II variant is a vaccine candidate for edema disease of swine. Infect Immun. 1992; 60: 485-490.
- Friedrich AW, Bielaszewska M, Zhang WL, Pulz M, Kuczius T, Ammon A, et al. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis. 2002; 185: 74-84.
- Melton-Celsa AR. Shiga Toxin (Stx) Classification, Structure, and Function. Microbiol Spectr. 2014; 2: 0024-2013
- Tseng M, Fratamico PM, Manning SD, Funk JA. Shiga toxin-producing Escherichia coli in swine: the public health perspective. Anim Health Res Rev. 2014; 15: 63-75.
- Beutin L, Krause G, Zimmermann S, Kaulfuss S, Gleier K. Characterization of Shiga toxin-producing Escherichia coli strains isolated from human patients in Germany over a 3-year period. J Clin Microbiol. 2004; 42: 1099-1108.
- Sonntag AK, Bielaszewska M, Mellmann A, Dierksen N, Schierack P, Wieler LH, et al. Shiga toxin 2e-producing Escherichia coli isolates from humans and pigs differ in their virulence profiles and interactions with intestinal epithelial cells. Appl Environ Microb. 2005; 71: 8855-8863.
- Fasel D, Mellmann A, Cernela N, Hachler H, Fruth A, Khanna N, et al. Hemolytic uremic syndrome in a 65-Year-old male linked to a very unusual type of stx2e- and eae-harboring O51:H49 shiga toxin-producing Escherichia coli. J Clin Microbiol. 2014; 52: 1301-1303.
- Thomas A, Cheasty T, Chart H, Rowe B. Isolation of Vero cytotoxin-producing Escherichia coli serotypes O9ab:H- and O101:H-carrying VT2 variant gene sequences from a patient with haemolytic uraemic syndrome. Eur J Clin Microbiol Infect Dis. 1994; 13: 1074-1076.
- Shimizu T, Ohta Y, Noda M. Shiga toxin 2 is specifically released from bacterial cells by two different mechanisms. Infect Immun. 2009; 77: 2813-2823.