
Research Article
Austin Immunol. 2016; 1(2): 1009.
Reestablishment of the Physiologic Tolerogenic Milieu after a Gut Inflammation is Time Dependent
Pereira e Silva A1,2, Campos SMN1,2, Pedruzzi MB1,2, Guimaraes IM1,2, De Mattos TM1,2, Vericimo MA1,2 and Teixeira GAPB1,2*
¹Departamento de Imunobiologia, Instituto de Biologia, Brazil
²Programa de Pós-Graduaço em Patologia, Hospital Universitário Antonio Pedro, Brazil
*Corresponding author: Gerlinde Agate Platais Brasil Teixeira, Departamento de Imunobiologia, Instituto Biologia, UFF, Alameda Barros Terra s/n, Centro, Niterói, RJ, Brazil
Received: September 16, 2016; Accepted: November 07, 2016; Published: November 09, 2016
Abstract
Aim: As new foods introduced to an inflamed gut may result in sensitization, our aim was to assess the time lapse required for recovery of the physiological milieu in order to, once again, develop tolerance to new proteins after a gut inflammation.
Methods: Male C57BL/6 (n=30) were immunized with 100μg-peanut protein followed by a 30-day chow (controls - C) or raw-peanut (experimental-EXP) challenge diet. On day 30 each group was split in three and received sweetened Egg White (EW) (20%-egg white, 5%-sucralose, H2Ov/v/v) orally as of day 0 (EXP-1/CONT-1), day 10 (EXP-2/CONT-2) or day 20 (EXP-3/CONT-3) postchallenge diet for 7 days. Body weight, food intake, antibodies, T cell phenotype of mesenteric lymph nodes (MLN) and spleen were assessed.
Results: OVA consumption was significantly lower in EXP-1 (3.2±1.0ml) compared to EXP-2 (6.5±1.19ml), EXP-3 (6.8±2.3ml) and CONT (7.5±1.7ml). MLN showed a significant increase of CD8+T cells in EXP-1 (29.5±4.1%) and EXP-2 (31.72±4.0%) compared to EXP-3 (21.5±3.6%) and CONT (25.7±5.4%) with no significant difference in CD4+T cells and CD4+CD25+T cells. Splenic CD4+T cells increased in EXP-1 (38.67±2.5%) compared to EXP- 2 (25.93±3.48%), EXP-3 (24.48±5.9%) and CONT (26.25±12.2%). Only EXP-1 showed gut inflammation after oral OVA challenge diet, presenting increased intraepithelial leukocytes, villi destruction/flattening and decreased goblet cells. EXP-2 and EXP-3 were similar to CONT.
Conclusion: In our model, 10 days is the time lapse required for the recovery of an inflamed gut to develop tolerance to novel proteins.
Keywords: Gut inflammation; Oral tolerance; Regulatory T cells; Food allergy
Abbreviations
AB: Antibody; Al(OH)3: Aluminum Hydroxide; ANOVA: Analysis of Variance; CD: Challenge Diet; CEUA: Comite de Etica na Utilizacao Animal; CONT: Allergic Control Group; ELISA: Enzyme-Linked Immunosorbent Assay; EXP: allergic Experimental group; EW: Egg White; HE: Hematoxilin-Eosin; HRP: Horseradish Peroxidase IEC/IEL: Intestinal Epithelial Cells per Intraepithelial Leukocytes; IgG or IgE: Immunoglobulin G or E; MALT: Mucosal Associated Immune Tissue; OIT: Oral Immunotherapy; OPD: O-Phenylenediamine; OVA: Ovalbumin; PBS: Phosphate Buffered Saline; v/v: volume per volume
Introduction
The most common ingredients used in human nutrition are widely tolerated by the general population. However, 4-8% of humans present some type of food allergy [1]. In theory, any food can cause allergic reactions, leading to harmless or severe life-threatening symptoms. Yet, over 90% of the medical reports are related to eight foods, called the “Big 8” allergenic foods, which include cow’s milk, crustaceans, fish, eggs, ground nuts, tree nuts, soy, and wheat [2]. In our multicultural society, with greater access to traveling and new food trends, the diversity of consumed food has broadened and therefore, so has the risk of developing food hypersensitivities [2,3]. Although the term “food hypersensitivity” was coined as an umbrella-term to incorporate both clinically diagnosed and self-reported adverse reactions to food including food allergies and intolerance [4,5], this term has not been accepted by the mainstream medical community. In this paper we will use the term “food allergy”, as proposed by the European Academy of Allergy and Clinical Immunology. “All hypersensitivity reactions initiated by immunologic mechanisms mediated by humoral (IgG or IgE) and/or cellular mechanisms should be named food allergy” [5-11].
The impact of food allergy may be greater than previously reported. A randomized, cross-sectional survey [12] evidenced that approximately 8.7% of the surveyed children presented food allergy. Among these, 38.7% presented severe-reactions and 30.4% multiplefood allergies. Although most of what is eaten is digested and is absorbed at the mucosal layer of the gastrointestinal tract as amino acids, glycose and small lipids (which are immunologically inactive), part of what is eaten is absorbed as molecules able to interact with the Mucosal Associated Immune Tissue (MALT) harbored in the gut. MALT is a complex and tightly regulated system that distinguishes innocuous from potentially harmful antigens, leading to either mucosal tolerance or systemic sensitization. An intense activation of the local immune system is necessary to obtain either outcome. Although a variety of T and B lymphocytes, including those with a regulatory profile and the epithelial barrier (intact or disrupted) are involved, the exact mechanisms governing this regulation remain to be elucidated [13-16]. During contact with specific antigens the establishment of tolerance induces a non-inflammatory response concomitant with low levels of systemic specific antibodies. Conversely, sensitization and food allergy leads to acute and/or chronic inflammation with high levels of allergen-specific antibodies [17,18]. Common clinical manifestations associated to food allergy are diarrhea, asthma, otitis, rhinitis, urticaria, dermatitis and eczema; laryngeal edema and anaphylactic shock [11]. Diagnosis is not obvious and the detection of food-specific antibodies does not always translate in clinical allergy. Therefore, a better understanding of the pathogenic mechanisms is strongly needed [19].
In experimental conditions, mucosal tolerance or allergy to novel food-proteins can be obtained by its introduction in the diet of an animal with a normal or inflamed gut, respectively implying that timing can be more important than allergenicity for the induction of food allergies [20,21]. Submitting allergic but not tolerant or normal animals to a challenge diet containing the corresponding allergen induces inflammatory architectural alterations of the gut mucosa [20,22,23]. Considering that the first contact with novel-diet proteins most often occurs in the early stages of life and that if this first contact occurs during an inflammatory gut process, it can lead to sensitization and not tolerance, an adequate timing of the introduction of novel diet-proteins is of high relevance in the clinical setting [20]. Thus, in this study our aim was to evaluate the necessary recovery time-lapse from an intestinal inflammatory process in order to safely introduce new diet-proteins and develop oral tolerance and not sensitization (allergy).
Material and Methods
Animals
Adult male C57BL/6 mice bred in the local Animal Facility of Universidade Federal Fluminense (originally obtained from the Jackson Laboratory in the 70’s) were given free access to food and water. Body weight was evaluated weekly. To perform paired statistical analysis all animals were individually numbered.
Food
According to the experimental protocol, (Figure 1) animals received commercial mouse chow (C-CD) (Nuvilab CR1 - NUVILABNUVITAL ®) without peanut or egg white proteins, Egg White diluted in Distilled Water 1:5 v/v, with 5% sucralose (EW-CD), Peanut in Natura Challenge Diet (P-CD), Ovalbumin Challenge Diet (O-CD). Food intake was measured three times a week and the mean caloric intake was calculated per gram of body weight per cage.

Figure 1: Timeline of the experimental protocol.
Immunization protocols
Animals were immunized subcutaneously twice, (21-day interval), with 100μg of the specific protein (preparation as described by Campos [23] [Peanut Protein Extract (PPE) or Ovalbumin (OVA)] with (primary) or without (booster) adjuvant [1mg of Al(OH)3].
Induction of the antigen-specific inflammatory gut reaction
After PPE immunization, animals were randomly divided into 2 groups (n=18 per group): allergic-control group (CONT), which continued to receive C-CD, allergic-experimental group (EXP) which received P-CD for 30 days.
Egg white feeding
Mice of the CONT and EXP groups were subdivided into three groups (n=6 per group) on the day of P-CD withdrawal and reintroduction of C-CD to the EXP-group. Water and EW were offered daily in separate drinking bottles for voluntary intake during 7 consecutive days starting on: day 0 for the EXP-1/CONT-1 groups; day 10 for EXP-2/CONT-2 groups and day 20 for EXP-3/CONT-3 groups. After 7 days of OVA intake, half of each group received a lethal dose of anesthetic for specimen collection (intestinal segments, spleen and mesenteric lymph nodes). The remaining half was submitted to the OVA immunization protocol followed by 30-days of O-CD (Figure 1).
Bleeding
Animals were bled 200μl from the retrorbital plexus 14 days after each immunization and at the end of the CD periods. The sera were collected and stored at -20°C until analyses.
Determination of Ab levels
Enzyme-linked immunosorbent assays (ELISA) were performed to detect specific IgG. Briefly, 96-well microplates (Alfa. Brazil) were coated with 4μg of antigen in 0.1M PBS per well overnight at 4°C, then blocked with 1% PBS-gelatin for one hour. Serum samples were added and a threefold serial dilution was performed. After 3h of incubation at room temperature detection was performed with goat anti-mouse IgG HRP (as recommended by Sigma-Aldrich®) and OPD (o-Phenylenediamine. Sigma-Aldrich®). The reaction was interrupted with 0.1M sulphuric acid and read at 492 nm using a microplatereader (Thermo Plate® – model 4200108). Results correspond to the area under the dilution curve of each serum.
Determination of T and B lymphocyte profile
At the different end-points (7, 17 or 27 days after P-CD removal or 30 days of O-CD) mesenteric lymph nodes (MLN) and the spleen of each animal were collected. Cells were washed, and re-suspended at 2x107 cells/ml in PBS and surface stained with αTCD4, αTCD8, αCD25, αFoxp3 and/or αB220 according to the manufacturer’s (Biolegend®) instructions. After running the samples on BD®-C6 Flow Cytometer, the activated lymphocyte region was determined. This was the gate in which all the remaining analysis were performed to evaluated TCD4+, TCD8+, TCD3+CD25+, TCD4+CD25+Foxp3+, TCD8+CD25+Foxp3+ and B-B220+CD25+ Lymphocytes populations.
Histomorphometry
At the different end-points intestinal segments were collected from each animal. These were immediately fixed with 10% buffered formaldehyde (PBS + 10% formaldehyde. Sigma-Aldrich®) processed and stained with Hematoxylin-Eosin (HE) or Alcian Blue. The histological sections were scanned with an Aperio® system and then analyzed with the aid of Imagescope software to quantify the histological parameters of the duodenum. For the purpose of this study the integrity of the intestinal architecture, the number of villi per 4000μm of intestinal tissue, mean villi area, leukocyte infiltrate and goblet cells per villus was analyzed. The ratios between villi height/width and intestinal epithelial cells/intraepithelial leukocytes (IEC/IEL) were then established.
Statistical analysis
Statistical analysis was performed using GraphPad InStat software by GraphPad Software Inc® and PASW Statistic software by Polar Engineering and Consulting Inc®. Mean ± Standard Deviation (SD), Pearson Correlation Test and ANOVA with Bonferroni or Tukey post-test were used. The minimum significance difference was considered for P value <0.05.
Results and Discussion
We observed an irregular behavior between groups on the first day of EW consumption. Half of the groups, irrespective of the gut condition, (EXP-1, EXP-3 and CONT-1) drank only small amounts (between 1-4 ml/group) while the other groups drank 3-7 fold of EW. As of the second day, groups with no inflammatory disease (CONT-1 and CONT-3), which started with small amounts, steadily increased their EW consumption while the group with an inflamed gut (EXP-1) maintained a low consumption profile (between 2-4ml/day) (Figure 2).
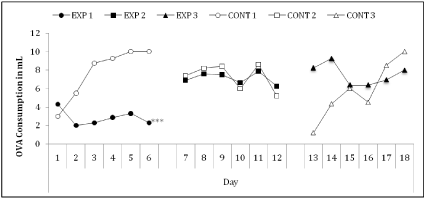
Figure 2: Daily consumption of egg white solution (ml/group (n=6)) by inflamed and control groups. The EXP-1 group drank significantly less solution than the
other groups (p<0.001) (Data from 1 experiment).
During the challenge diet period, irrespective of the diet (C-CD or P-CD), neither allergic nor control mice gained or lost significant weight. However, during EW intake, after removal of P-CD and reintroduction of C-CD, EXP-1 group presented a significant (p<0.05) weight loss (-1.03±0.98g), while the EXP-2 (4.16±2.03g) and EXP-3 (2.93±1.59g) groups gained more weight than control groups (1.57±0.62g). During the immunization period, no significant alterations in weight were observed. Nevertheless, after the 30-day O-CD period a significant weight loss (p<0.01) was observed in EXP- 1 (-8.71±3.67g) (p<0.001) and EXP-2 (-2.75±1.24g) groups while EXP-3 (1.58±0.81g) and CONT (1.04±0.45g) groups continued to gain weight (Figure 3).
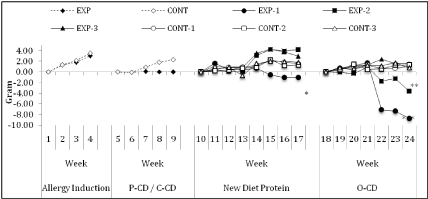
Figure 3: Means of weekly animal weight in each group in grams. The EXP did not gain weight as compared to the CONT group in the P-CD. The EXP-1 group lost
significantly more weight when compared to the EXP-3 and CONT groups in the new protein period (p<0.05). The EXP-1 and EXP-2 group lost significantly more
weight when compared to the EXP-3 and CONT groups in the O-CD period (p<0.001 and p<0.01, respectively). The EXP-3 group showed no significant differences
to the CONT groups during the experiment (Data from 1 experiment).
Macroscopic analysis of the abdominal cavity
The macroscopic examination of the peritoneum of all EXP groups during the P-CD recovery period showed a pale coloring of the organs and a frail consistency of the intestinal tissue in contrast to the control groups. These characteristics were less intense in the EXP- 2 and EXP-3 groups. After the O-CD, the macroscopic examination of the peritoneum showed a pale coloring of the organs and frail consistency of the intestinal tissue only of EXP-1 group. All other groups presented normal macroscopic morphology.
Evaluation of total IgG titers
While anti-peanut IgG titers were high in both EXP (3.85±0.59) and CONT (3.92±0.92) groups, anti-OVA IgG antibodies were high only in the EXP-1 group (3.35±1.21) (p<0.001), EXP-2 (0.54±0.16), EXP-3 (1.27±0.39) and CONT (1.11±0.42) (Figure 4).
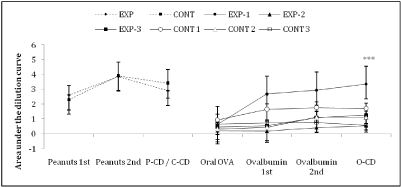
Figure 4: Area under the dilution curve of each serum anti-Peanuts and anti-OVA IgG titers. We observed no differences between the EXP and CONT groups after
sensitization and P-CD. After sensitization with OVA, the EXP-1 group showed higher anti-OVA antibodies titers when compared to the other inflamed and control
groups (p<0.001) (Data from 1 experiment).
Lymphocyte profile
After the 30-day O-CD period: although not significant, more TCD3+CD25+ than B-B220+CD25+ lymphocytes were retrieved from both the spleen and MLN of all groups (Figure 5A). Comparing, the MLN T cell compartment between groups, a significantly higher percentage was observed in the EXP-1 (0.106±0.02%) (p<0.01) when compared to the EXP-2 (0.074±0.010%), EXP-3 (0.076±0.011%) and CONT (0.060±0.011%). The same comparison for the splenic TCD3+CD25+ population showed no significant differences (Figure 5A). The percentage of MLN TCD4+CD25+Foxp3+ population was significantly higher (p<0.05) in the EXP-2 (0.15±0.01%) and EXP-3 (0.15±0.02%) when compared to EXP-1 (0.12±0.02%) and CONT (0.12±0.009%) groups. No significant difference was observed in the splenic TCD4+CD25+Foxp3+ population (Figure 5B). No Fopx3 expression was observed within the TCD8+CD25+ cells from either MLN or spleen.
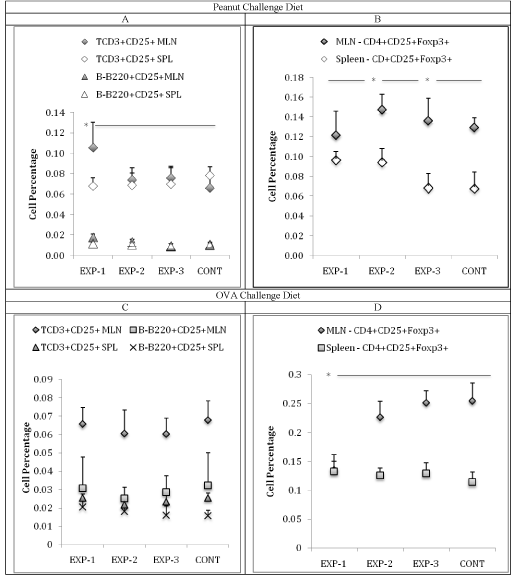
Figure 5: (A) Mean of T-CD3+CD25+ and B-B220+CD25+ cell percentage of the EXP and control group in mesenteric lymph nodes and spleen. In MLN, the EXP-1
group showed higher T-CD3+CD25+ cells when compared to the other groups (p<0.01). In the spleen we observed no differences between the groups. (B) Mean
of TCD4+CD25+Foxp3+ cell percentage of the Inflamed and control group in mesenteric lymph nodes and spleen. In MLN, the EXP-2 and EXP-3 groups showed
higher TCD4+CD25+Foxp3+ cells when compared to the other groups (p<0.05). In the spleen we observed no differences between the groups. (C) After OVA CD:
Mean of T-CD3+CD25+ and B-B220+CD25+ cell percentage of the EXP and control groups in mesenteric lymph nodes and spleen. No significant differences were
observed. (D) Mean of TCD4+CD25+Foxp3+ cell percentage of the EXP and control groups in mesenteric lymph nodes and spleen. In the spleen we observed
no significant differences between the groups, but in the MLN the EXP-1 showed a significant decrease (p<0.05) when compared to the EXP-2, EXP-3 and CONT
groups, that were similar to each other (Data from 1 experiment).
After the 30-day O-CD period
The T cell population is larger than the B cell in the MLN, but not in the spleen of all groups. No inter-group differences were observed in the TCD3+CD25+ population (Figure 5C). However, significantly less (p<0.05) TCD4+CD25+Foxp3+ cells were retrieved from the MLN of the EXP-1 (0.13±0.01%) group compared to all other groups (EXP- 2 (0.22±0.01%), EXP-3 (0.25±0.01%) CONT (0.25±0.01%)) with no significant inter-group differences in splenic cells (Figure 5D).
Microscopic analysis of the intestine
After oral EW: The mean villus area of the CONT groups (2.5x104±0.47x104μm2) is significantly higher (p<0.001) when compared to all EXP groups. No significant difference was observed in between EXP-1 (1.9x104±0.53x104μm2); EXP-2 (2.0x104±0.67x104μm2); EXP-3 (2.1x104±2.8x103μm2), although a positive time vs. area correlation exists (Table 1). The number of villi per 4000μm of duodenum was significantly smaller (p<0.01) in the EXP-1 (12.6±1.5) when compared to all other groups. Although without significant difference between the remaining groups, a steady time-dependent growth of villi number occurs (Correlation = p<0.05) i.e. EXP-2 (16.7±1.2), EXP-3 (17.5±2.3) and CONT (20.3±2.1) (Table 1). The villi height/width ratio, of CONT (4.83±0.66), was significantly higher (p<0.01) than EXP-1 (1.7±0.64) (p<0.001); EXP- 2 (3.08±0.57) (p<0.01); and EXP-3 (3.48±0.27) with a time dependent recovery correlation (p<0.05) (Table 1).
Parameter
Sensit.
EXP-1
EXP-2
EXP-3
CONT
Histomorphometry
Number of Villi
PEANUTS
12.6±1.5
16.7±1.2
17.5±2.3
20.3±2.1
OVA
19.6±4.0
19.5±3.5
19.0±3.6
17.0±3.2
Area (μm2x102)
PEANUTS
193.1±53.0
200.0±66.8
215.6±28.3
253.7±46.6
OVA
174.3±5.6
204.4±9.7
213.4±25.1
199.8±26.4
Height / Width
PEANUTS
1.7±0.64
3.08±0.57
3.48±0.27
4.83±0.66
OVA
2.98±0.02
3.88±0.51
3.74±0.04
3.45±0.20
IEC / IEL
PEANUTS
27.3±12.8
42.5±21.6
45.2±23.4
71.8±27.7
OVA
23.6±4.75
34.23±3.06
39.57±6.96
40.63±2.26
Number of Goblet Cells
PEANUTS
2.95±0.52
3.24±0.27
3.76±0.50
4.26±0.45
OVA
3.65±0.13
7.32±0.17
7.36±0.48
6.76±0.18
Summary showing the comparison between the two stages of Histomorphometric analysis. Bold values mean statistically significance when compared to the control group (p<0.05). EXP-1: Received OVA on 1st week after P-CD. EXP-2: Received OVA on 2nd week after P-CD. EXP-3: Received OVA on 3rd week after P-CD. Then, all groups were immunized with OVA and received the O-CD (Data from 1 experiment).
Table 1: Comparison between the two stages of histomorphometric analysis.
After O-CD: the mean villus area of the EXP-1 group (1.7x104±0.56x104μm2) was significantly smaller (p<0.05) when compared to the CONT (1.9x104±0.26x104μm2), EXP-2 (2.4x104±0.97x104μm2) and EXP-3 (2.1x104±0.25x104μm2) (Table 1). No significant differences intra and inter-group were observed for the number of villi per 4000μm in the duodenum (EXP-1: 19.6±4.0; EXP-2: 19.5±3.5; EXP-3: 19.0±3.6) and CONT: 17.0±3.2 (Table 1). The height/width ratio of the villi of the EXP-1 (2.98±0.02) was significantly smaller (p<0.01) when compared to the CONT (3.45±0.20), EXP-2 (3.88±0.51), EXP-3 (3.74±0.04) groups (Table 1).
After oral EW, the number of IEC per villus did not vary significantly within groups. Nevertheless, the number of IEL correlates positively with the intensity of the inflammatory process, i.e. IEL count is higher in the most inflamed groups (data not shown). Thus, when compared to the CONT group (1.97±0.10) the most significant decrease in the IEC/IEL ratio per villus was observed in the EXP-1 (1.26±0.30) (p<0.01) followed by EXP-2 (1.59±0.30) and EXP-3 (1.61±0.33) groups (p<0.05) with a positive time dependent recuperation correlation (p<0.05) (Table 1). The same positive time dependent correlation was observed for goblet cells per villus. EXP-1 (2.95±0.52) and EXP-2 (3.24±0.27) presented significantly lower goblet cells (p<0.01) than the EXP-3 (3.76±0.50) and CONT (4.26±0.45) groups.
After the O-CD, no changes in the IEC count were observed and IEL only increased in the group that received EW during the first week of P-CD removal (data not shown), thus affecting the IEC/IEL ratio of the EXP-1 (23.6±4.75) (p<0.05) which is significantly lower than EXP-2 (34.23±3.06), EXP-3 (39.57±6.96) and CONT (40.63±2.26) groups (Table 1). As for the goblet cells, again only EXP-1 (3.65±0.13) group presented significantly lower goblet cell count, (p<0.001). EXP- 2 (7.32±0.17), EXP-3 (7.36±0.48) and CONT (6.76±0.18) (Table 1).
(Table 2) depicts the comparison of the histomorphometric analysis between each inflamed group when compared to the control group after the P-CD and recovery weeks, thus giving a clear sight of time-dependent recovery. (Figure 6) shows the intestinal mucosa of each group after P-CD, C-CD and O-CD.
Villi/field
Area
Height/Width
IEC/IEL
Number of
Goblet Cells
INFL 1
ê
Same as CONT
ê
ê
ê
INFL 2
Same as CONT
Same as CONT
ê
ê
ê
INFL 3
Same as CONT
Same as CONT
ê
ê
Same as CONT
The arrows indicate statistically significance when compared to the control group (p<0.05). EXP-1: Received OVA on 1st week after P-CD. EXP-2: Received OVA on 2nd week after P-CD. EXP-3: Received OVA on 3rd week after P-CD.
Table 2: Summary of Intestinal Parameters of the inflamed groups compared to the control group after P-CD.
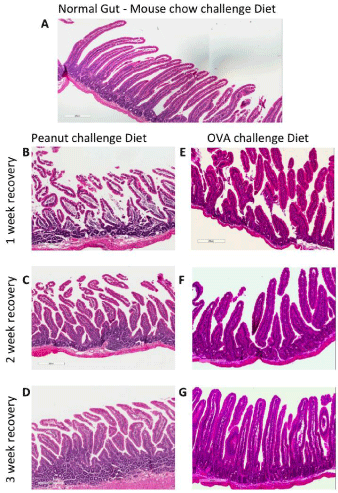
Figure 6: Representative photomicrographs of the small intestine showing a time-dependent improvement after removal of the challenge diet (CD) and a time
dependent correlation of sensitization to a non-related antigen. (A) Control group - peanut allergic animals after mouse-chow CD. (B, C, D) Peanut allergic animals
one, two or three weeks after removal of peanut challenge diet respectively (P-CD) (B) after one-week of P-CD removal there is a great destruction of the villi,
presence of edema and cell infiltrate. (C) After the two-weeks of P-CD removal an improvement of the villi architecture can be seen (less edema). (D) After threeweek
of P-CD removal an almost normal duodenum with minor IEL number is observed. (E, F, G) peanut allergic animals that ingested OVA during the 1st, 2nd
and 3rd week after removal of P-CD and were submitted two a second challenge diet containing OVA (O-CD). (E) OVA ingestion during the 1st week post-P-CD
removal shows that O-CD induced a discrete inflammatory process. (F) And (G) OVA ingestion during the 2nd and 3rd week post-P-CD removal respectively does
not sensitize the animals and the O-CD did not inflame the gut. These were considered normal tissues (Data from 1 experiment).
The similarity of the kinetics between animal food allergy models and human patients has attracted research interest [24]. Knowing that: 1) the introduction of an unknown protein in an inflamed gut induces a new allergy characterized by a systemic sensitization [20] and 2) that in physiological conditions, the entry of food in the gut induces tolerance, we proposed to determine the necessary recoverytime after the removal of pro-inflammatory agents in order to regain the ability to induce oral tolerance to new proteins. We began our analysis with the assessment of the sensitization process. All animals showed similar levels of anti-peanut antibody titers, comparable to previous work of our group [23], indicating that a successful allergy induction to peanuts was achieved. Although this is not the natural route by which allergy is normally established, for experimental protocols it is a strategy that permits the exact quantification of the amount of antigen to be introduced. As previously established, high and low doses of antigens tend to trigger different lymphocyte profiles and the induction of a predominance of IgE [24,25] or IgG [26,27] allergy. In our model we use a high antigen dose and encounter predominantly specific IgG in the C56BL6 mice that are Th1 prone [28]. As for allergic humans, in our experimental model high antibody titers are not enough for the induction of gut inflammation. Thus, no inflammation of the gut develops either in the allergic animals that do not eat the challenge diet or in celiac patients that do not eat gluten or its derivatives.
However, when the offending protein (challenge diet containing exclusively the allergenic protein) is introduced in the diet of individuals producing high antibody levels a time-dependent inflammatory process of the gut develops. As previously described, for humans [29] and mouse [20] this inflammation is characterized by an initial hypertrophy due to cell infiltration, followed by villi widening and shortening with a concomitant edematous process. If the offending stimulus continues, the flattening of the villi may be observed. Other pathological processes such as hemorrhage, enlargement of the Peyer’s patches, alteration in the goblet cell numbers and mucous production are also observed with an increasing time-dependent intensity. This is the typical scenario for nontreated human Celiac Disease and for the antigen specific chronic gut inflammation described by our group [20,30]. The opposite is also true: after removal of the antigenic stimulus (offending diet component), recovery is also time dependent, as seen in this work and in the clinical setting [31].
Our results suggest that a tolerance induction milieu is not regained immediately after the removal of the offending antigens. A recovery period is necessary before the introduction of new diet proteins induce tolerance again. Those animals that ingested EW as the newly introduced diet protein during the first week after P-CD removal presented significantly higher anti-OVA antibody levels when compared to all other groups (EW ingestion during the 2nd and 3rd week after P-CD removal and those who ingested EW with no gut inflammation), suggesting a systemic sensitization even though these animals ingested significantly less EW than animals of the 2nd and 3rd week. We argue that the introduction of a novel protein while the animals are still feeling sick may induce an aversion to the new food. However, the small amount of ingested protein was sufficient to induce systemic sensitization. These data are in accordance with previous studies that showed that systemically sensitized mice [32,33] or mice with an antigen specific gut inflammation present aversion to the corresponding food [18]. On the other hand, we can also argue that the replacement of an offending diet with a diet that causes wellbeing (diminishing inflammation) may initially deviate the animal’s attention from the unknown new food, although they avidly drink sweetened EW in physiological conditions [32]. Thus, as all control animals (with a normal gut) and those groups that had access as of the 2nd week drank all the offered EW, we inferred that the residual inflammation was no longer effective in deviating the animals’ liking for EW. Taking these data together, it is possible to argue that the link between the immunological and the nervous systems may act as a protection against the development of multiple allergies.
The regulation of the immune response in our experimental model is mediated with a predominance of T cells over B cells in both mesenteric lymph nodes and spleen, as shown by flow cytometry after the 30-day P-CD. On the other hand, we observed similar percentages of T and B cells after the O-CD. These data agree with those of Noh [34], which showed that there is an increase of the regulatory T cell population in both inflammatory and tolerant milieus in the spleen of patients sensitized to casein. Although the literature has tried to elucidate the role of B-lymphocytes in the regulatory responses, further studies are still needed to correlate their actions with the induction and maintenance of oral tolerance and food allergies. In the MLN we found a significant increase of T cells in the EXP- 1 group when compared to the other groups. Although this change may seem small numerically, it is proven to be biologically significant as observed by other works in the literature, since TCD3+CD25+ cells are just a small percentage of the whole T cell population in the body [35,36].
The Histomorphometric analysis of the gut showed that the intensity of the inflammatory parameters are inversely proportional to the time elapsed after removal of the P-CD, reaching almost normal parameters within three weeks, agreeing with previous studies [23,37,38]. The clinical implications for these findings are that the inflammatory milieu may be one of the triggers of food allergies. Thus, the correct timing of food introduction after a gut inflammation (allergic or infectious) is of great importance, especially in children that may become sensitized and therefore can develop allergy to new foods [39].
The exclusion of the offending allergens in allergic individuals with gut inflammation appears to be important. In fact, the most effective strategy for the recovery of the mucosa in active celiac disease is total gluten exclusion. The recovery of the patients’ body weight, general state and biochemical parameters can be observed as the inflammatory process decreases [40,41]. As previously shown [37] in our experiments, we find the same kinetics. In this work we suggest that the presence of dietary Tolerogenic substances may accelerate recovery of the gut mucosa.
Correlating the state of the intestinal mucosa and the consequences of the introduction of the new food showed interesting results. Our findings presented here show that in our mouse model, during the 1st week of mucosal recovery, it is not possible to induce oral tolerance. Instead, a new allergy was established. However, after a week of recovery, allergy is no longer induced. We hypothesize that a shift in the cytokine profile from a Th1/Th2 to a regulatory or Th3 response rapidly occurs and we believe that the return to a TGF-β rich milieu is responsible for the reestablishment of tolerance induction. We are currently investigating this hypothesis. Another fact that we still need to determine is the exact amount of time that is needed after the removal of an inflammatory insult to reestablish the physiological reactivity to food from the immunological standpoint. These facts can be of great relevance to the clinical setting and especially to pediatricians, since most foods are introduced for the first time in the diet during early childhood, after weaning and when the mothers’ protection is no longer present.
Conclusion
As conclusion, in this gut inflammation model, 10 days is the time-lapse required for the recovery of an inflamed gut to develop tolerance to novel proteins.
Acknowledgment
The Integrated Unit of Specialized Pathology, associated to the Pathology Graduate Program, Department of Pathology at Federal Fluminense University.
The authors would like to thank Maira Platais for helping with the English translation. We also thank CNPq and Capes for the grants given that made this work possible.
Disclosure
All authors disclose that there are no financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work.
References
- White BL, Shi X, Burk CM, Kulis M, Burks AW, Sanders TH, et al. Strategies to mitigate peanut allergy: production, processing, utilization, and immunotherapy considerations. Annual Review of Food Science and Technology. 2014; 5: 155-176.
- Zukiewicz-Sobczak WA, Wroblewska P, Adamczuk P, Kopczynski P. Causes, symptoms and prevention of food allergy. Postepy Dermatol Alergol. 2013; 30: 113-116.
- Husain Z, Schwartz RA. Food allergy update: more than a peanut of a problem. International Journal of Dermatology. 2013; 52: 286-294.
- Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001; 56: 813-824.
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. The Journal of Allergy and Clinical Immunology. 2004; 113: 832-836.
- Page-Reeves J. Conceptualizing Intersecting Dynamics, Disjunctures and Disparities in the Experience of Food Allergy. Food, Culture & Society. 2015; 18: 5-29.
- Tanno LK, Calderon MA, Goldberg BJ, Akdis CA, Papadopoulos NG, Demoly P. Categorization of allergic disorders in the new World Health Organization International Classification of Diseases. Clinical and Translational Allergy. 2014; 4: 42.
- Soares-Weiser K, Takwoingi Y, Panesar SS, Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy. 2014; 69: 76-86.
- Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010; 303: 1848-1856.
- Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014; 69: 62-75.
- Lifschitz C, Szajewska H. Cow’s milk allergy: evidence-based diagnosis and management for the practitioner. European Journal of Pediatrics. 2015; 174: 141-150.
- Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States. Pediatrics. 2011; 128: 9-17.
- Mayer L. Mucosal immunity and gastrointestinal antigen processing. Journal of Pediatric Gastroenterology and Nutrition. 2000; 30: 4-12.
- Steele L, Mayer L, Berin MC. Mucosal immunology of tolerance and allergy in the gastrointestinal tract. Immunologic Research. 2012; 54: 75-82.
- Lied GA. Indication of immune activation in patients with perceived food hypersensitivity. Digestive Diseases and Sciences. 2014; 59: 259-266.
- Karlsson MR, Johansen FE, Kahu H, Macpherson A, Brandtzaeg P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy. 2010; 65: 561-570.
- Cardoso CR, Teixeira G, Provinciatto PR, Godoi DF, Ferreira BR, Milanezi CM, et al. Modulation of mucosal immunity in a murine model of food-induced intestinal inflammation. Clin Exp Allergy. 2008; 38: 338-349.
- Teixeira G, Paschoal PO, De Oliveira P, Pedruzzi MMB, Campos SMN, Andrade L, et al. Diet selection in immunologically manipulated mice. Immunobiology. 2008; 213: 1-12.
- Sampson HA. Update on food allergy. The Journal of Allergy and Clinical Immunology. 2004; 113: 805-819.
- Paschoal PO, Campos SM, Pedruzzi MM, Garrido V, Bisso M, Antunes DM, et al. Food allergy/hypersensitivity: antigenicity or timing? Immunobiology. 2009; 214: 269-278.
- Cabrera CM, Urra JM. Food allergy and the oral immunotherapy approach. Arch Immunol Ther Exp (Warsz). 2015; 63: 31-39.
- Antunes DM, Da Costa JP, Campos SM, Paschoal PO, Garrido V, Siqueira M, et al. The serum D-xylose test as a useful tool to identify malabsorption in rats with antigen specific gut inflammatory reaction. International journal of experimental pathology. 2009; 90: 141-147.
- Campos SM, De Oliveira VL, Lessa L, Vita M, Conceicao M, Andrade LA, et al. Maternal immunomodulation of the offspring's immunological system. Immunobiology. 2014; 219: 813-821.
- Oyoshi MK, Oettgen HC, Chatila TA, Geha RS, Bryce PJ. Food allergy: Insights into etiology, prevention, and treatment provided by murine models. The Journal of Allergy and Clinical Immunology. 2014; 133: 309-317.
- Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. The Journal of Clinical Investigation. 2006; 116: 833-841.
- Khodoun MV, Strait R, Armstrong L, Yanase N, Finkelman FD. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proceedings of the National Academy of Sciences of the United States of America. 2011; 108: 12413-12418.
- Berin MC, Sampson HA. Food allergy: an enigmatic epidemic. Trends in Immunology. 2013; 34: 390-397.
- Palumbo ML, Canzobre MC, Pascuan CG, Rios H, Wald M, Genaro AM. Stress induced cognitive deficit is differentially modulated in BALB/c and C57Bl/6 mice: correlation with Th1/Th2 balance after stress exposure. Journal of Neuroimmunology. 2010; 218: 12-20.
- Lahdeaho ML, Maki M, Laurila K, Huhtala H, Kaukinen K. Small- bowel mucosal changes and antibody responses after low- and moderate-dose gluten challenge in celiac disease. BMC Gastroenterology. 2011; 11: 129.
- Tennyson CA, Lewis SK, Green PH. New and developing therapies for celiac disease. Therapeutic Advances in Gastroenterology. 2009; 2: 303-309.
- Stoven S, Murray JA, Marietta E. Celiac disease: advances in treatment via gluten modification. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. 2012; 10: 859-862.
- Cara DC, Conde AA, Vaz NM. Immunological induction of flavour aversion in mice. II. Passive/adoptive transfer and pharmacological inhibition. Scandinavian Journal of Immunology. 1997; 45: 16-20.
- Mirotti L, Mucida D, De Sa-Rocha LC, Costa-Pinto FA, Russo M. Food aversion: a critical balance between allergen-specific IgE levels and taste preference. Brain, Behavior, and Immunity. 2010; 24: 370-375.
- Noh J, Noh G, Kim HS, Kim AR, Choi WS. Allergen-specific responses of CD19(+) CD5(+) Foxp3(+) regulatory B cells (Bregs) and CD4(+) Foxp3(+) regulatory T cell (Tregs) in immune tolerance of cow milk allergy of late eczematous reactions. Cellular Immunology. 2012; 274: 109-114.
- Ban H, Andoh A, Shioya M, Nishida A, Tsujikawa T, Fujiyama Y. Increased number of FoxP3+CD4+ regulatory T cells in inflammatory bowel disease. Molecular Medicine Reports. 2008; 1: 647-650.
- Lau KM, Cheng SH, Lo KW, Lee SA, Woo JK, van Hasselt CA, et al. Increase in circulating Foxp3+CD4+CD25(high) regulatory T cells in nasopharyngeal carcinoma patients. British Journal of Cancer. 2007; 96: 617-622.
- Campos SM. Avaliacao do Efeito da Tolerancia Oral na Recuperacao da Inflamacao Intestinal Cronica. Niteroi: Universidade Federal Fluminense. 2013.
- Pedruzzi MMB. Influencia da administracao da 1,25 vitamina D3 na inflamacao intestinal cronica antigeno-específica. Niteroi - Rio de Janeiro: Universidade Federal Fluminense. 2013.
- Vickery BP, Scurlock AM, Jones SM, Burks AW. Mechanisms of immune tolerance relevant to food allergy. The Journal of Allergy and Clinical Immunology. 2011; 127: 576-584.
- Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001; 120: 636-651.
- Niewinski MM. Advances in celiac disease and gluten-free diet. Journal of the American Dietetic Association. 2008; 108: 661-672.