
Review Article
Austin Immunol. 2018; 3(1): 1013.
The Microsphere Fluorescent Antioxidant (MFA) Assay: Antioxidant Activity of Spices
Jerry T. Thornthwaite, Kathleen Hamrick and Elise Chaffin
Cancer Research Institute of West Tennessee, USA
*Corresponding author: Jerry Thornthwaite, Cancer Research Institute of West Tennessee, Henderson, TN, USA
Received: December 18, 2017; Accepted: February 08, 2018; Published: February 15, 2018
Abstract
Increased production of reactive oxygen species is a feature of most, if not all, human disease, including cardiovascular disease and cancer. Dietary antioxidants may be especially important in protecting against human diseases associated with free radical damage to cellular DNA, lipids, and proteins. Yet, present data are not sufficient to quantify micro nutrient requirements needed to protect against oxidative damage. The antioxidant roles of many food constituents, including herbs and spices, have not been clarified. The flow cytometric Microsphere Fluorescent Antioxidant (MFA) assay measures the oxidation of R-Phycoerythrin (PE) attached to 5.0μM microspheres via a protein coupling linker. In order to form the Radical monocation, a 25mM stock solution of ABTS (2,2’-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid free radical) was incubated 12 hr at 4C with potassium persulfate to cause removal of one electron generating a metastable ABTS cation radical (ABTS+•). This stock solution was diluted to 100μM in 50ul, which resulted in 80% quenching of 15μl of 1x104 PE microspheres. The antioxidant effects were measured by first incubating the 80% quenching ABTS+• with100μl portions of serial diluted antioxidants for 30 min. at RT to determine the antioxidants and concentrations that neutralized the ABTS+• Subsequently,15μl of PE-tagged microspheres were added to each sample and incubated one hour at RT. The peak fluorescent channel number was determined for 5,000 microspheres. The typical percentage protection by the antioxidant standard at 500ng/ml for Trolox (water soluble Vitamin E derivative) was 82%±0.02 SD, while Vitamin C restored 78%±0.01SD of the protective effect. For the range of 500-5,000ng/ ml, the protective effects of Trolox and Vitamin C remained constant. On the average, equivalent percentage protection for the lipid soluble fractions of 10 spice types with duplicates (courtesy of McCormick Science Institute) was not achieved until the 2,050 μg/ml levels with Cloves, Ginger, and Oregano showing the highest antioxidant protection. Interestingly, Rosemary, Sage, Thyme and Turmeric revealed a significant reduction in antioxidant activity at 2,050μg/ml. These are still significant antioxidant levels that show a secondary reason to use spices for achieving a measurable antioxidant activity. The MFA assay is very sensitive to the effects of oxidants, requires little sample, and allows application to the study of serum samples. Therefore, the antioxidant activity in healthy volunteers taking compounds to boost the antioxidant activity and to study the effects of oxidative radiation and chemotherapy may be monitored.
Introduction
Oxidation and reduction reactions occur naturally in the body and work together to maintain equilibrium in many biochemical pathways. Oxidation reduction (redox) reactions are processes in which electrons are transferred from one chemical species to another. Electrons are lost during an oxidation reaction and electrons are gained in a reduction reaction. When a molecule is oxidized, another compound must gain the electron and become reduced. Therefore, redox reactions occur simultaneously to maintain a balance of electrical charge. A common biological example of oxidation and reduction occurs during the energy production process, cellular respiration. The energy produced is the result of a series of redox reactions. An example of a redox reaction is in the overall reaction of aerobic cellular respiration in utilizing glucose to produce ATP, the biological energy currency. In the overall reaction, glucose is oxidized to produce carbon dioxide, and oxygen is reduced to produce water as shown in the following chemical equation C6H12O6+6O2→6CO2+6H2O+36ATP.
There is a delicate balance between oxidation and reduction in cellular respiration and other biochemical processes. During metabolism, all oxygen consuming cells create intermediates and byproducts. These molecules are referred to as Reactive Oxygen Species (ROS) [1,2]. ROS are naturally occurring biological free radicals. Free radicals are molecules which have a single, unpaired electron in the valence shell. Free radicals and ROS are highly reactive, unstable molecules that seek another electron to fill the valence shell. In order to stabilize ROS, the body performs complimentary reduction reactions by using biological antioxidants. These molecules buffer against oxidation and fill electron shells, preventing the buildup of ROS [3].
There is significant literature linking polyphenol-rich foods like fruits, vegetables, and spices to the prevention of cancer, cardiovascular disease, diabetes, and cognitive degeneration [4- 7]. These diseases are also linked with oxidative damage; therefore, the antioxidant properties are thought to be responsible for disease protection [8-10]. Since there is a correlation, it is important to determine the most effective antioxidants and the mechanisms by which they operate.
Despite the presence of endogenous antioxidant systems, oxidative damage can accumulate over time. Numerous studies have shown the intake of antioxidants can provide health benefits [11]. Dietary antioxidants in particular have consistently been associated with health benefits. Frequent consumption of antioxidant-rich foods such as fruits, vegetables, and spices has been shown to decrease the risk of the diseases linked with oxidative damage. For example, a study analyzed the diets of over 900 men and women. Individuals who consistently consumed an antioxidant-rich diet showed considerably lower glycemic index levels, putting them at a much lower risk for developing type II diabetes [12]. Furthermore, antioxidant-rich medicinal plants significantly inhibited enzymes that promote high blood pressure and high blood sugar, both of which are risk factors for type II diabetes [13].
Population studies have revealed a correlation in antioxidant food consumption and disease risk. Asian populations, who regularly consume more fruits and vegetables than populations with a western diet, exhibit a lower incidence of cancers [14]. In vitro studies have shown polyphenols present in fruits and vegetables inhibit cancer cell growth and tumor angiogenesis, while promoting cancer cell death [15]. A study of 800 elderly men showed daily consumption of fruits, vegetables, and tea led to increased plasma antioxidant levels and positive cardiovascular health [16]. The chemical components and physiological mechanisms by which these foods combat disease are still being researched. However, most studies attribute health benefits to a group of compounds called polyphenols. Polyphenols are very stable molecules with multiple phenol rings which are able to neutralize free radicals. Studies have revealed a strong positive relationship between antioxidant capacity and phenol content [17,18].
There is a large amount of literature linking polyphenol-rich foods like fruits, vegetables, and spices to the prevention of cancer, cardiovascular disease, diabetes, and cognitive degeneration. These diseases are also linked with oxidative damage; therefore, the antioxidant properties are thought to be responsible for disease protection. Since there is a correlation, it is important to determine the most effective antioxidants and the mechanisms by which they operate. The purpose of this study is to develop a sensitive, accurate, and multifunctional assay to study antioxidant compounds which can be applied to biological testing. The new assay will build upon principles established in previous testing methods and introduce flow cytometry and microsphere technology as the means of analysis. The assay should yield results comparable to accepted methods and prove to be an effective tool in antioxidant research.
Materials and Methods
Preparation of the target fluorescent microspheres
The Microsphere Fluorescent Antioxidant (MFA) assay uses the BD Accuri flow cytometer (BD Biosciences) to measure the fluorescent quenching of Phycoerythrin bound to 5.0μM carboxylated polystyrene spheres. As shown in Figure 1, the MFA assay utilizes polystyrene microspheres (Bangs Lab) with carboxyl chemical groups. The carboxyl group allows for easy attachment via the EDAC linker (Bangs Lab) to the primary amine groups of the R-RPhycoerythrin (PE) protein (Sigma-Aldrich). PE is a light harvesting protein from the phycobiliprotein family isolated from marine algae [19,20]. The protein can be used as a label or probe when conjugated to microspheres, antibodies, or biomolecules in a cell [21]. PE is one of the most useful and versatile fluorochromes, because it has abroad excitation spectrum and is significantly brighter and more stable than other fluorescent proteins [22]. The optimal absorption is in the bluegreen range of the visible spectrum between 490 and 560nm and the emission spectrum is above 610nm in the orange- red range [23].

Figure 1: EDAC coupling with R-Phycoerythrin (Protein) to the 5.0 μm
carboxylated polystyrene microspheres.
The PE-coupled 5.0μm microspheres emit orange-red fluorescence when excited by the 488nm blue laser as shown by the fluorescent microscopy image in Figure 2. The fluorescence can be quantified by the flow cytometer. The uninhibited fluorescent microspheres serve as a reference standard for the quenching fluorescence using the MFA assay.
Figure 2: Fluorescent image of the R-Phycoerythrin-labeled 5.0μm
microspheres.
The microspheres were prepared according to the procedure in the PolyLink coupling kit. In order to prepare the fluorescent microspheres, 0.0980g of the 5μm polystyrene microspheres were weighed in a 2.5ml micro centrifuge tube. The microspheres were centrifuged for 8min. at 3500rpm (1500xg) at 4C and the supernatant was removed. A 60ml portion of the Polylink Coupling Buffer (PCB) was added to re suspend the microspheres, and they were centrifuged again under the same conditions. After removing the supernatant, the microspheres were resuspended in 1.36ml of PCB. Just before addition, 0.0400g of Polylink EDAC was weighed and dissolved in 200μl of PCB. 160μl of EDAC solution was then added to the microspheres. Immediately after adding EDAC, 20μg of R-phycoerythrin (R-PE) was also added. The solution was allowed to incubate for one hour at room temperature in the dark with gentle, periodic mixing. After incubation, the solution was centrifuged at 4C for 10 minutes at 3500rpm (400xg) and the supernatant was removed. The microspheres were resuspended in 2ml of Polylink Wash Buffer (PWB). The centrifuge process was repeated two more times to ensure excess protein and EDAC were removed [Polylink datasheet]. The microspheres were stored in 2ml of PWB at 4C in the dark at were stable at least 30-60 days. Before use, the solution was diluted with wash buffer until a rate of about 300 events per second was recorded on the flow cytometer.
Preparation of control oxidant, ABTS+•
ABTS (Sigma-Aldrich) is the common name for the compound 2, 2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid). The radical cation form (ABTS+•) is made by reacting potassium persulfate with ABTS in water. Upon the addition of the ABTS+•, the PE fluorescence of the microspheres will be quenched in direct proportion to the concentration of the ABTS. When oxidized, PE exhibits proportionally diminished fluorescence resulting in a lower peak channel number. In all of these studies, the ABTS+• is quenched by the antioxidants before exposure to the PE labeled microspheres. The antioxidant works by reducing both the ABTS+• to ABTS, which does not quench the PE fluorescence.
The ABTS+• was prepared by weighing out 0.0685 g of ABTS and adding 2.5ml of water to create a 50mM ABTS solution. A 150mM solution of potassium persulfate was prepared by dissolving 0.1015g of potassium persulfate in 2.5ml of water. Both the ABTS+ solution and persulfate solution were combined and incubated at 4C for 12hrs in order for the radical cation to stabilize [24]. Before use, 100μl of ABTS+• solution was diluted in 1.5ml of water. A 180μl portion of this solution was diluted with 13ml of pure water. Through experiment, it was determined that 50μl of the final dilution will cause about 80-90% inhibition of PE fluorescence, which is an ideal percentage inhibition range to measure inhibition recovery.
Trolox is the antioxidant standard
Trolox is the antioxidant standard for the assay and is considered to be the gold standard in antioxidant testing. Trolox (Cayman Chemical Co.) is the trade name for the water-soluble vitamin E analog, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. All other antioxidants tested in the assay are compared to the antioxidant activity of Trolox. Fluorescence of the microspheres will more closely approach the original, uninhibited fluorescence (increased peak channel number) as the strength of the antioxidant increases. The exponential quenching of ABTS+• is directly proportional to the effectiveness of the antioxidant used in these studies.
The Trolox was prepared by dissolving 0.0025g of Trolox in 1ml of acetone, which was diluted to 50ml with pure water. Before the experiment, the solution was diluted by 110 to create a solution with 500ng of Trolox per 100μl. The diluted acetone had no effect on the MFA Assay.
Spice extracts were prepared by McCormick® Spices
Spice extracts were prepared by McCormick® Spices and were provided as concentrated extract solutions. Fourteen spice samples were made in alipophilic solutions for a total of 14 samples. Approximately 0.5g of each sample was accurately weighed and was extracted with two 5ml portions of methylenechloridehexane (50:50 v/v) solution. The extraction solutions were combined and the solvent was removed using a Turbovap concentrator. The resulting residue was redissolved in 10.0ml of acetone, creating a concentrated solution for lipophilic. antioxidant analysis. Solutions were stored at 4C to maintain stability. The extract solutions require dilution prior to the experiment due to the sensitivity of the flow cytometer. Following dilution, the average concentration was about 500μg of spice per 100μl. Aqueous dilution for both sample types is acceptable. For this study, 10 lipophilic samples, some in duplicate, were selected to show the utility of the MFA assay.
Experimental procedure
The standard protocol for the assay is as follows. A 96- well microtiterplate is used to contain the samples for the test. The preparation procedure ensures an equal volume of solution in each sample well of the microtiter plate. In the first well, 200μl of properly diluted Trolox is added. For the remaining wells in the top first row, 200μl of desired spice extracts are added in a concentration matching the Trolox. A 100μl portion of pure water is added to the remaining wells of the microtiterplate. Serial dilutions are performed by removing 100μl from the first row of wells and mixing the solution with the 100μl of water in the second row of wells. A 100μl portions was removed from each well in the second row and mixed with the water in the third row. The procedure is repeated until all eight rows have been diluted. In the eighth row, 100μl are removed and discarded. By performing this procedure, each sample contains half the amount of antioxidant after each dilution. To each well 50μl of ABTS+• was added and allowed to incubate with the antioxidant for a few minutes. After the solutions are prepared, 15μl of Phycoerythrinlabeled microspheres are added to each sample well. The samples are incubated at room temperature (22-24C) for 1 hour in the dark. After incubation, the samples are analyzed in the red fluorescent PE channel using the Accuri C6 flow cytometer and C Flow Plus software package (BD Biosciences).
By running the flow cytometer in kinetic mode, as shown in Figure 3, the oxidant effect of ABTS+• on the PE labeled microspheres rapidly attenuates the fluorescence. The addition of Trolox supplies an electron per ABTS+• causing recovery of PE fluorescence within seconds.
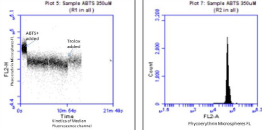
Figure 3: Kinetics of the quenching of the PE labeled microspheres by
ABTS+. And recovery of fluorescence (antioxidant activity) by the Trolox.
Second panel shows the PE fluorescence flow cytometry distribution of the
PE labeled microspheres.
Before testing the samples, a blank solution of microspheres is run to determine the original, uninhibited fluorescence peak fluorescent channel. A software gate is applied to the population representing the majority of the microspheres. The gate eliminates outliers from the sample reading and removes background readings from extraneous particles which may be in the solution. A new graph can be made to record the average fluorescence for each sample, and the gate applied to all graphs and data readings. The FL-2 (red) channel is used to measure fluorescence, because the 488 nm solid state laser emission can excite the PE fluorochrome. Each sample is run on the flow cytometer until 3,000 events are recorded inside the gated area. The average FL-2 peak for these events is recorded. All sample measurements are compared to the original uninhibited fluorescence and all spice sample measurements are compared to Trolox. After all samples have been analyzed, the Trolox and microsphere blank are recorded a gain to check for stability of the standard Trolox curve before and after the typical two-hour analysis time frame (Figure 4).
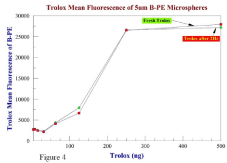
Figure 4: Stability of the Trolox standard curve fresh and two hrs later at
room temperature.
Results
In Tables 1 and 2, the peak channel fluorescent channels are shown to verify the precision of the MFA assay Preliminary experiments were completed to determine effective concentrations of ABTS•+ for inhibiting microsphere fluorescence. ABTS•+ showed to be a very effective oxidant, in quenching even strong fluorescence by 50 % at 300μM. The concentration of ABTS•+ used provides significant inhibition to allow antioxidants to demonstrate protection.
In (Table 2, Figures 4, and 5), the range of Trolox antioxidant recovery (vitamin C was similar, Table 1) was with a maximum recovery Range of 200-300 ng of Trolox.
TROLOX*
TX FL
SPICES*
1L Basil
2L B.Pepper
3L Cinn.
4L Cinn.
5L Cloves
6L Ginger
.5 μg
28295
200 μg
14263
17894
8476
8919
23182
22628
0.25 μg
25031
100 μg
18052
18706
8585
9895
24956
24248
0.125 μg
15069
50 μg
6227
10251
3014
5900
24279
23642
0.0625 μg
11982
25 μg
4344
7116
3287
3850
24503
24402
0.031 μg
8887
12.5 μg
4054
4631
3260
3312
22628
23423
0.016 μg
6566
6.25 μg
3065
3590
2531
3318
22342
15210
0.008 μg
3570
3.13 μg
3072
3097
2878
3026
22774
6359
0.004 μg
2967
1.56 μg
2212
2131
2208
2150
21819
2766
Sum Fluoresc
55291
67416
34239
40369
186482
142679
TX 1hr**
7L Ginger
8L Oregano
9L Oregeno
10L R.Pepper
Beads Start
Beads 1hr
.5 μg
25957
200 μg
22411
15724
14148
23429
29647
26059
0.25 μg
22614
100 μg
23460
20397
19288
24506
0.125 μg
10947
50 μg
23253
21282
20009
20887
0.0625 μg
9320
25 μg
23837
22232
21221
14322
0.031 μg
7057
12.5 μg
23649
16998
21436
5452
0.016 μg
5445
6.25 μg
20317
7122
19937
4007
0.008 μg
2925
3.13 μg
10346
3718
13125
3471
0.004 μg
2568
1.56 μg
3289
2346
3351
2267
Sum Fluoresc
150560
109819
132514
98340
Table 1: Comparisons between Trolox (TX) and Vitamin C (VC) in four separate experiments peak fluorescence channels are shown.
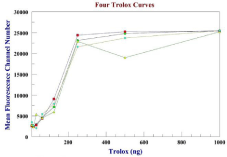
Figure 5: Four Trolox standard curves using the MFA assay. The typical
percentage protection by the antioxidant standard at 500 ng/ml for Trolox was
82% ± 0.02 SD n=4.
Maximum recovery for the lipid soluble spices varied between 50 to less than 2μg/well (Table 2, Figures 6 and 7). For the spices, (Figure 6) maximum antioxidant activity was seen with Cloves, Ginger, and Red Pepper. The antioxidant recovery with Trolox at fresh and 1-2 hrs after analyses can be seen in Table 1 and 2. The stability of the MFA assay is shown in shown in Figure 4 at two hours.
Amount
5000 ng
2500ng
1250ng
625ng
313ng
156ng
78.1ng
39.0ng
19.5ng
9.8ng
4.9ng
2.4ng
VC1
32,141
33,678
34,462
34,192
32,562
10,509
5,353
3,756
3,204
3,099
3,347
3,534
VC2
33,575
33,407
34,528
34,167
32,271
9,786
5,516
3,215
3,450
3,087
3,288
3,128
VC3
31,881
32,220
33,029
33,084
31,969
9,078
4,725
3,560
3,264
3,213
3,117
3,139
VC4
32,080
29,668
30,569
33,530
32,728
7,275
4,103
3,216
3,093
3,074
3,042
3,438
Mean Vit C
32,419
32,243
33,147
33,743
32,383
9,162
4,924
3,437
3,253
3,118
3,198
3,310
TX1
34,857
33,958
34,935
35,147
21,895
7,774
4,616
3,483
3,519
2,832
3,269
3,068
TX2
35,415
34,988
35,039
35,341
25,442
7,310
4,500
3,395
3,178
3,000
3,116
3,219
TX3
33,900
34,477
34,981
34,964
20,408
7,255
4,219
3,368
3,309
2,897
3,175
3,210
TX4
33,921
34,448
34,616
34,416
23,952
7,603
4,777
3,279
3,283
3,281
3,276
3,393
Mean TX
33,354
34,468
34,893
34,967
22,924
7,485
4,528
3,381
3,322
3,002
3,209
3,222
Table 2: TROLOX Standard Antioxidant recovery vs. Lipid fraction SPICES MFA assay results with average peak fluorescence of the PE quenching and recovery with antioxidant. The sum fluorescence is used to generate bar graphs in Figure 7.
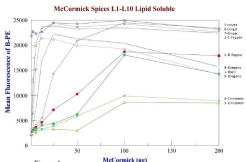
Figure 6: Antioxidant recovery curves for 10 preparations of lipid soluble
spices. Compared to each other, cloves>Ginger>Red Pepper showed the
strongest antioxidant activity.
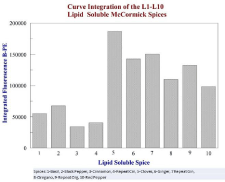
Figure 7: Curve integration of Figure 6 showing the spices with the
highest oxidative activity, which is a summation of antioxidant activity at all
concentrations.
Trolox and vitamin C were significantly superior antioxidants than any of the spices as shown in Table 1, and 2, where Trolox (vitamin E) and vitamin C, were very effective in the ng/ml range vs. μg/ml range for the spices.
The sum fluorescence over the entire range of concentrations was recorded with each experiment to quantify which spice allowed the most overall fluorescence, which signified the strongest antioxidant to reverse the oxidant effect of the ABTS+•. Ideally, the spice which demonstrates the most average fluorescence over the concentration range would be the most effective antioxidant. For the lipophilic samples, cloves showed significantly better fluorescence among the species. The results shown in Figure 6 indicate that each spice sample may have an optimal range for antioxidant activity. In several of the experiments, the highest concentration of antioxidant did not show the greatest amount of fluorescence protection. At high concentrations in vitro, some antioxidants show pro-oxidant behavior, which may or may not occur in vivo.
Overall, the lipophilic-derived spice fraction was more consistent in showing antioxidant activity than the hydrophilic-derived spices (data not shown). Part of the reason for this was the hydrophilic spice extracts were prepared in a solution containing acetic acid. While the concentration of acetic acid is small, this assay is sensitive to low concentrations of acid. The acid may have reacted with the PE, causing inhibition at high spice concentrations. Furthermore, PE has been shown to perform better as a fluorochrome in lipophilic environment [25].
PE-labeled microspheres are stable for at least six weeks when stored at 4C. None of the experiments showed significant variation between initial and final fluorescent experimental readings (Table 2 and Figure 4).
Trolox and vitamin C patterned closely to one another as seen in Table 1. Both provided significant protection from oxidant concentrations in the nmol range. The low concentrations used in these experiments demonstrate the sensitivity of the flow cytometry MFA assay. Trolox and vitamin C are accepted as potent antioxidants. The typical percentage protection by the antioxidant standard at 500ng/ml for Trolox (water soluble Vitamin E derivative) was 82% ±0.02 SD n=4, while Vitamin C restored 78% ± 0.01SD n=4 of the protective effect. For the range of 500-5,000ng/ml, the protective effects of Trolox and Vitamin C remained constant.
Discussion
The purpose of this study is to develop a sensitive, accurate, and multifunctional assay to study antioxidant compounds which can be applied to biological testing. The new assay builds around flow cytometry and microsphere technology as the means of analysis. The assay yields results comparable to accepted methods and proves to be an effective tool in antioxidant research.
Studies relating spice consumption to health benefits are surprisingly difficult because dietary intake is hard to quantify. Food surveys often do not account for spice consumption because spices are used as seasoning ingredients in a meal. Spice usage varies highly in manufactured and prepared food, and home use is typically random and prepared taste. The USDA has data on the inventory and production of herbs and spices in the United States, which can provide a rough estimate on consumption. As of 2006, the estimated per-capita spice consumption was 3.3 pounds per year. This was a 2 pound per person increase since 1966 [26]. Analysis studies on cultural recipes have been conducted to estimate spice consumption for certain populations. In United States recipes, an average of 5 spices was used per dish. Usage ranged from 1.6 spices per recipe in Norway to 6.9 spices per recipe in Indonesia and other Asian countries [27]. Although specific data is difficult to obtain, there are relationships between spice consumption and disease incidence. A population study in Singapore revealed men and women age 60-93 demonstrated better cognitive performance on a mental health exam where turmeric is consumed regularly in diet [28]. Another study found the country of Georgia to have a low incidence of colorectal cancer, despite the high meat content of the Georgian diet. The result is thought to be caused by the liberal use of spices in meat processing and preservation. The study measured the antioxidant capacity of the Georgian spices and found high antioxidant activity and phenolic content in spices like caraway, barberry, and red pepper [29].
Laboratory studies have also revealed potential health implications for spice consumption. Curcumin, derived from the dietary spice turmeric, has been found to have profound anti-cancer properties [8,9,30]. One study in particular showed that curcumin inhibited the proliferation of K562 leukemia cells by inducing cell death [9]. Curcumin was not studied in the water soluble fraction in this study because of the acetic acid problem in the MFA assay. However, the literature clearly shows curcumin has antioxidant activity and directly toxic to cancer cells [8,9]. Cinnamon is a powerful antioxidant which has been linked to the prevention of type II diabetes and cardiovascular disease. A laboratory study was performed on rats with high cholesterol. Rats who were given cinnamon along with cholesterol medication exhibited higher levels of endogenous serum antioxidants, nitricoxide, and high-density lipoproteins (“good cholesterol”) than untreated rats. These results indicate cinnamon supplementation improved cardiovascular function in the test rats and could help prevent cardiovascular disease [31]. Red Pepper was evaluated for the contents of different antioxidants compounds and their antioxidant activities in jalapeno peppers. The antioxidant activity assay showed that the ElD or ido and Grande had strongest antioxidant activity [32]. Clove (Syzygiumaromaticum) is one of the most valuable spices that has been used for centuries as a food preservative and for many medicinal purposes. Clove is native to Indonesia, but now is cultured in several parts of the world including Brazil in the state of Bahia. This plant represents one of the richest sources of phenolic compounds such as eugenol, eugenolacetate and gallic acid [33]. Ginger has antioxidant properties that might be attributed to their hydroxyl groups and suitable solubilizing sidechains [34]. Oregano has been widely used in folk medicine to alleviate inflammation-related diseases, respiratory and digestive disorders, headaches, rheumatism, diabetes and others. These potential health benefits are partially attributed to the phytochemical compounds in oregano such as flavonoids and phenolic acids [35].
The principles outlined for the new assay have been established as effective through previous testing techniques. The most widely accepted and referenced antioxidant assay technique is the Oxygen Radical Absorbance Capacity (ORAC) assay [36]. The ORAC assay, first developed in 1993 [37], measures the change of fluorescence intensity of a fluorescent probe when exposed to different concentrations of oxidants and antioxidants. The original protocol used beta-phycoerythrin as the fluorescent protein and Trolox as the antioxidant standard. The oxidant was AAPH (2,2’-azobis(2- amidino-propane) dihydrochloride),a molecule similar to ABTS [38]. The ORAC assay procedure has remained relatively unchanged, but other fluorescent probes such as fluoresce in are commonly used [39]. Solutions containing fluorescent probes are prepared an uninhibited sample is scanned to obtain original fluorescence. The oxidant AAPH is added to the solution. Over time, AAPH quenches fluorescence, but the addition of Trolox help store store fluorescence to the probes. Fluorescence is measured by a spectrophotometer, a technique used to measure the intensity of light at a certain wave length. ORAC units are used to quantify antioxidant activity. One ORAC unit is equal to the net protection provided by 1μmol of Trolox [37-39]. The majority of studies, including those referenced in this study, have used the ORAC approach to determine antioxidant activity.
Other antioxidant assays have utilized the ABTS radical cation. The experimental methods for this oxidant have typically included spectrophotometry and decolorization, not fluorescence [40,41]. The technique has been criticized, because the antioxidant reaction proceeded at a faster rate than the oxidation reaction, reducing the system before any changes were observed. While the assay showed the protective properties of antioxidants, no measurable value was given for antioxidant activity [42]. With the sensitivity of fluorescent flow cytometry, we have determined a better technique in which the ABTS is already stabilized in radical form before being added to a system. This allows antioxidants to demonstrate the ability to neutralize pre existing free radicals [42]. The proposed flow cytometry assay utilizes the pre-formed radical cation species of ABTS.
The data presented in this paper provide proof of principle for this new MFA assay. The studies have shown significant precision and accuracy in comparing antioxidant activity. The use of fluorescencebased flow cytometry has increased sensitivity and resolution. Thus far, most antioxidant assays have been performed entirely in vitro without using human serum or cells. While the preliminary testing is performed in vitro, the flow cytometry assay may be modified to test the antioxidant activity in human serum. Our results from previous studies (data not shown) suggest the assay could work well under physiological conditions. The measurement of antioxidant activity in serum after eating antioxidant-rich meals, will enable a better understand how dietary antioxidants react in the body. The use of microspheres and flow cytometry introduces versatility because multiple parameters can be tested using a single sample. Microspheres also allow for the attachment of antibodies to detect specific biomarkers in human serum. Antibodies can be made to specifically detect endogenous antioxidants in serum such as super oxidedismutase, glutathione eperoxidase, and catalase. They can also be made to indicate oxidant and antioxidant activity in specific parts of a cell, such as the mitochondria. A single sample may contain microspheres of various sizes for getting protocols in which each size microsphere is labeled with specific antibodies and fluorochromes for analyses from a single sample.
Acknowledgement
We thank the McCormick Science Institute for a grant and supplying the spices involved in this study. Special thanks to Dr. Tony Kirk and Bonita Thornthwaite for proof reading of this manuscript. Other funding has come from the Shumard and Carter Foundations.
References
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003; 552: 335-344.
- Turrens JF, Freeman BA, Levitt JG, Crapo JD. The effect of hyperoxia on super oxide production by lung submitochondrial particles. Arch Biochem. Biophys. 1982; 217: 401-410.
- Droge W. Free radicals in the physiological control of cell function Physiol Rev. 2002; 82: 47-95.
- Tang GY, Meng X, Li Y, Zhao CN, Liu Q, Li HB. Effects of Vegetables on Cardio vascular Diseases and Related Mechanisms. Nutrients. 2017; 9: 857.
- Yang Y, Karakhanova S, Werner J, Bazhin AV. Reactive oxygen species in cancer biology and anti cancer therapy. Curr Med Chem. 2013; 20: 3677- 3692.
- Balsano C, Alisi A. Antioxidant effects of natural bio active com pounds. Curr Pharm Des. 2009; 15: 3063-3073.
- Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998; 68: 1081-1087.
- Thornthwaite JT, Shah HR, Shah P, Peeples WC, Respess H. The formulation for cancer prevention & therapy. Adv Biol Chem. 2013; 3: 356-387.
- Thornthwaite JT, Shah HR, England SR, Roland LH, Thibado SP, Ballard TK, et al. Anticancer Effects of Curcumin, Artemisinin, Genistein, and Resveratrol, and Vitamin C: Free Versus Liposomal Forms. Adv Biol Chem. 2017; 7: 27-41.
- Thibado SP, Thornthwaite JT, Ballard TK, Goodman BT. Anticancer effects of Bilberry anthocyanins compared with Nutra Nano Sphere encapsulated Bilberry anthocyanins. Mol Clin Oncol. 2018; 8: 330-335.
- Balsano C, Alisi A. Antioxidant effects of natural bioactive compounds. Curr Pharm Des. 2009; 15: 3063-3073.
- Psaltopoulou T, Panagiotakos DB, Pitsavos C, Chrysochoou C, Detopoulou P, Skoumas J, et al. Dietary antioxidant capacity is inversely associated with diabetes biomarkers: The ATTIC A study. Nutr Metab Cardiovasc Dis. 2011; 21: 561-567.
- Ranilla LG, Kwon Y, A postolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bio resource Tech. 2010; 101: 4676-4689.
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. Bethesda, MD.
- Kandaswami C, Lee LT, Hwang JJ, Ke FC, Huang YT, Lee MT, et al. The anti tumor activities of flavonoids. In Vivo. 2005; 19: 895-909.
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993; 342: 1007-1011.
- Shan B, Cai Y, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005; 53: 7749-7759.
- Rodov V, Vinokur Y, Gogia N, Chkhikvishvili I. Hydrophilic and lipophilicanti oxidant capacities of Georgian spices for meat and their possible health implications. Georgian Med News. 2010; 179: 61-66.
- Kronick MN. The use of phycobili proteins as fluorescent labels in immunoassay. J Immunol Methods. 1986; 92: 1-13.
- Festin R, Björklund B, Tötterman TH. Detection of triple antibody-binding lymphocytes in standard single laser flow cytometry using colloidal gold, fluoresce in and phycoerythrin as labels. J Immunol Meth. 1987; 101: 23-28.
- Kronick MN, Grossman PD. Immuno assay techniques with fluorescent phycobili protein conjugates. Clin Chem. 1983; 29: 1582-1586.
- White JC, Stryer L. Photostability studies of phycobiliprotein fluorescent labels. Anal Biochem. 1987; 161: 442-452.
- Hardy RR. Purification and coupling of fluorescent proteins for use in flow cytometry Hand book of Experimental Immunology 4th. Black well Scientific Publications, Boston. 1986; 31: 1-31.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Bio Med. 1999; 26: 1231-1237.
- Zhao M, Sun L, Fu X, Gong X. Influence of ionic strength, pH, and SDS concentration on sub unit analysis of phycoerythrins by SDS-PAGE. Appl Biochem Biotechnol. 2010; 162: 1065-1079.
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. Bethesda.
- Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, et al. Health benefits of herbs and spices: the past, the present, the future. Med J Aust. 2006; 185: S1-24.
- Lee L, Kang SA, Lee HO, Lee BH, Park JS, Kim JH, et al. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health. 2001; 115: 133-138.
- Rodov V, Vinokur Y, Gogia N, Chkhikvishvili I. Hydrophilic and lipophilic antioxidant capacities of Georgian spices for meat and their possible health implications. Georgian Med News. 2010; 179: 61-66.
- Panda AK, Chakraborty D, Sarkar I, Khan T, Sa G. New insights in to therapeutic activity and anti cancer properties of curcumin. J Exp Pharmacol. 2017; 9: 31-45.
- Roussel AM, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J Am Coll Nutr. 2009; 28: 16-21.
- Farhoudi R, Mehrnia MA, Lee DJ. Anti oxidant activities and bioactive compounds of five Jalapeno peppers(Capsicum annuum) cultivars. Nat Prod Res. 2017; 1-4.
- Cortés-Rojas DF, deSouza CR, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014; 4: 90-96.
- Si W, Chen YP, Zhang J, Chen ZY, Chung HY. Antioxidant activities of ginger extract and its constituents to ward lipids. Food Chem. 2018; 239: 1117-1125.
- Gutiérrez-Grijalva EP, Picos-Salas MA, Leyva-López N, Criollo-Mendoza MS, Vazquez-Olivo G, Heredia JB. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants (Basel). 2017; 7.
- Bentayeb K, Vera P, Rubio C, Nerín C. The additive properties of Oxygen Radical Absorbance Capacity (ORAC) assay: the case of essential oils. Food Chem. 2014; 148: 204-208.
- Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants,” Free Radic Biol Med. 1993; 14: 303-311.
- Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998; 68: 1081-1087.
- Cao G, Russell RM, Lischner N, Prior RL. Serum anti oxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J Nutr. 1998; 128: 2383-2390.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Bio Med. 1996; 20: 933-956.
- Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA. Antioxidant activities of carotenes and xanthophylls. FEBSLett. 1996; 384: 240-242.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radicalcation decolorization assay Free Radic Bio Med. 1999; 26: 1231-1237.