
Case Report
Austin Immunol. 2021; 5(1): 1019.
Natural Killercell Deficiency (NKD)
Hussain M*, Tipu HN, Ahmed D, Alam M, Sheikh S, Ijaz Y
Department of Immunology, Armed Forces Institute of Pathology (AFIP), Pakistan
*Corresponding author: Muhammad Hussain, Department of Immunology, Armed Forces Institute of Pathology (AFIP), Rawalpindi, Pakistan
Received: February 12, 2021; Accepted: March 01, 2021; Published: March 08, 2021
Abstract
Natural Killer Cell Deficiency (NKD) alone is a rare disorder of innate immune system.Isolated NK cell is deficiency can be classical or functional. In classical Natural killer cell deficiency, NK cells are ≤1% of peripheral blood lymphocytes whereas functional NK cell deficiency is associated with decrease ability to perform its function. NKD makes patients more prone to viral infections (especially with Herpes, Varicella zoster, Cytomegalo & Ebsteinbarr viruses) and malignancies. This defect should be suspected in patients with repeated fever and chest infections, when all other causes of primary immunodeficiency are ruled out. A 2-years-female patient with repeated chest infections, on off fever since the age of 7 months with very poor to antibiotics and negative blood culture on several occasions. Lymphocyte subset analysis repeated at a gap of two months and a final diagnosis of Natural killer cell deficiency was established.
Keywords: Natural killer cells deficiency; Primary immunodeficiency; Viral infections
Introduction
Natural Killer Cell Deficiency (NKD) alone is a rare defect of innate immune defense. This is distinct from the NK cell abnormality which is present as a minor component in more than 50 primary immunodeficienciessuch as NK- SCID, Wiskott-Aldrich syndrome, Chediak-Higashi syndrome, Common Variable immunodeficiency, Familial Hemophagocytic lymphohistiocytosis and should not be labeled as NKD1.Natural killer cells are produced from hematopoietic stem cells in bone marrow and about 10% of NK cells are present in peripheral blood [2]. Isolated NK cell deficiency can be distinguished into classical and functional. In classical NKD, NK cells are low or absent that is ≤1% of peripheral blood lymphocytes whereas in functional defect, normal or increase numbers of NK cells are present with decrease ability to perform its function of destroying stressed cells and cells with absent MHC [2]. Natural killer cells perform their cytotoxic function by perforin and granzyme molecules and have main role in elimination of viral infection and surveillance of cancerous cells3. NKD makes patients more prone to viral infections especially herpes, CMV, EBV, papilloma and some virus associated malignancies3. So far 6 gene defects have been identified (Figure 1) and further research will make NKD more understandable, add in diagnosis and management of these patients [4,5]. The diagnosis is based primarily on Lymphocyte subset analysis of NK cells which lacks surface expression of CD16, CD56 and CD3 by flow cytometry, NK cell functional assays and molecular diagnosis [6].
Figure 1: Different mutations of NKD.
Case Presentation
Two years old female child, third baby of their parents, resident of Multan (Punjab) visited Immunology Department, Armed Forces Institute of Pathology (AFIP) Rawalpindi. The child presented with H/O of recurrent episodes of fever and chest infections since the age of 7 months. Umbilical cord separation occurred on 6th postnatal day. No history of abscesses, skin rash, diarrhea or mouth ulcers and she lost 3 kg weight in last one year. Child had poor response to antibiotics and received all vaccines till date. Her parents are first cousins. This patient have two elder sisters, both are healthy and achieved normal milestones on time. Her 3 maternal uncles were also healthy with no significant medical history. On examination, she looked malnourished with stable vitals, mild pallor, no cyanosis, mouth and skin ulcers. Liver and spleen were not palpable. The child was referred to AFIP Rawalpindi Immunology Department for Primary Immunodeficiency workup. Previous reports of this patient showed low Hb-8.8 g/dl with increase MCV, TLC 6.4x109/L (70% lymphocytes) and Platelets 84x109/L. Due to repeated lymphocytosis on complete blood counts, bone marrow aspiration was also performed which showed hypercellualr fragments, megakaryocytes but no blast cells were observed. For his decrease Hb, increase MCV and megakaryocytes presence in bone marrow, vitB12 and folic acid levels were performed which were found low. Serum ferritin was within upper limits of normal. Her serum Immunoglobulin level showed IgA 0.74 g/l (0.4-3.5), IgM 1.01 g/l (0.5-3), IgG8.77 g/l (6.5- 15) and IgE less than 150 IU/ml which was normal. HIV serology repeated twice on different occasions and was found negative. HRCT chest showed mild peribronchial wall thickening with no exudative lesion, lymphadenopathy or pleural fluid. Cystic fibrosis was also ruled out by sweat chloride test and F506 mutation analysis. Her lymphocytes counts showed normal T and B cells with very low number of NK cells that is 36 (normal range 300-600). After thorough history and considering the previous lab reports, following lab investigations were carried out at AFIP Rawalpindi including IgG subclasses, physiological antibodies response and lymphocyte subset analysis which showed IgG1 5.14 g/l (3.2-10.2), IgG2 1.14 g/l (1.2-6.6), IgG3 2.04 g/l (0.2-1.9), IgG4 0.15 g/l (0.1-1.5), Anti E coli and candida response was present and lymphocyte subset analysis showed absent NK cells with normal T and B cells population (Figure 2). To further confirm the diagnosis, viral antibodies profile was carried out at AFIP Rawalpindi which showed high titre of IgG antibodies, positive for Herpes simplex, varicella zoster, CMV and both IgM & IgG antibodies positive for EBV. On the basis of history, physical examination and lab investigations carried out at AFIP, final diagnosis of Natural Killer Cell Deficiency (NKD) was established. The functional NK cell assay not performed as NK cells were completely deficient and molecular diagnostic facility is not available in Pakistan. The patient parents were counseled and advised for viral prophylaxis and regular screening for viral associated malignancies of their child.
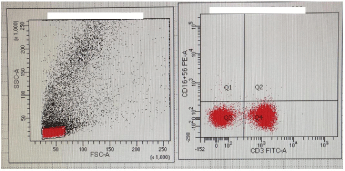
Figure 2: Flow cytometric analysis of CD3, CD16 and CD56. Left panel
shows gated population and right dotplot shows complete absence of CD16
and CD56 whereas CD3 having normal expression.
Discussion
Natural Killer Cell Deficiency (NKD) alone is anemerging identity of the primary immune deficiency which is further divided into classical natural killer cell deficiency with NK cells ≤1% of peripheral blood lymphocytes whereas in functional defect, normal or increase numbers of NK cells are present with decrease ability to perform its functions [7].
The first case of NKD was published in New England Journal of Medicine in 1989 in a young girl with multiple herpes and Cytomegalovirus infections. She was diagnosed by flow cytometric analysis which showed CD3 , CD56-, CD16- lymphocytes (absent NK cells) and cytotoxic activity as measured by K562 killing assays [8]. A total 19 patients studied with absence of NK cells in different primary immunodeficiency disorders which showed 42% (8/19) died early and 53% (10/19) experienced severe viral infections. In NK cell deficiency, patients are more prone to viral infections especially Herpes, Varicella zoster, Cytomegalovirus, Epstein barr & Human papilloma virus2.We also found positive antibody titre for Herpes simplex, Varicella zoster, Cytomegalovirus and Epsteinbarr virus in our patient. A large consanguineous Irish cohort study showed that 3 family members had ≤1% NK cells in peripheral blood. One of them had recurrent EBV driven lymphoproliferative disease. Molecular studies showed microsatellite homozygosity mapping and the affected locus was linked to chromosome 8 [9].
The diagnosis is based primarily on the lymphocyte subset analysis of Natural killer cells with lack of surface expression ofCD16, CD56, CD3 by flow cytometry, NK cell functional assay, viral antibodies profile for different viruses and molecular diagnosis with exclusion of all other primary immune deficiencies. Treatment options include viral prophylaxis, cytokine therapy, bone marrow transplant and gene therapy is under trial. This is one of the rare case of primary immunodeficiency in which Natural killer cells are only absent and makes patient more prone to viral infections. This case of Natural Killer Cell Deficiency (NKD) is not reported in this region and being reported for the first time in Pakistan.
References
- Orange JS. How I Manage Natural Killer Cell Deficiency. Journal of Clinical Immunology. 2020; 40: 13-23.
- Orange JS. Natural killer cell deficiency. Journal of Allergy and Clinical Immunology. 2013; 132: 515-525.
- Moon WY, Powis SJ. Does Natural Killer Cell Deficiency (NKD) increase the risk of cancer? NKD may increase the risk of some virus induced cancer. Frontiers in immunology. 2019; 10: 1703.
- Ebbo M, Gerard L, Carpentier S, Vely F, Cypowyj S, Farnarier C, et al. Low circulating natural killer cell counts are associated with severe disease in patients with common variable immunodeficiency. E Bio Medicine. 2016; 6: 222-230.
- Mace EM, Orange JS. Emerging insights into human health and NK cell biology from the study of NK cell deficiencies. Immunological reviews. 2019; 287: 202-225.
- Eguizabal C, Herrera L, Ingles-Ferrandiz M, Belmonte JI. Treating primary immune deficiencies with defects in NK cells: from stem cell therapy to gene editing. Stem Cell Research & Therapy. 2020; 11.
- Lam MT, Mace EM, Orange JS. A research-driven approach to the identification of novel natural killer cell deficiencies affecting cytotoxic function. Blood, The Journal of the American Society of Hematology. 2020; 135: 629-637.
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. New England Journal of Medicine. 1989; 320: 1731-1735.
- Eidenschenk C, Dunne J, Jouanguy E, Fourlinnie C, Gineau L, Bacq D, et al. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. The American Journal of Human Genetics. 2006; 78: 721-727.