Abstract
Hyponatremia frequently complicates management of Congestive Heart Failure (CHF), either due to disease severity and/or due to diuretic use. It is an independent predictor of increased morbidity and mortality in CHF. Evidence suggests that there is significant variability among health care professionals in the understanding of pathophysiology and management of hyponatremia in hospitalized CHF patients. Adequate management of hyponatremia in CHF may reduce in hospital mortality and CHF related hospital readmission rate. This article will review the mechanism of hyponatremia in CHF; its prognostic implications; and the available evidence based management strategies.
Keywords: Hyponatremia; Congestive heart failure; Ultrafiltration; Arginine vasopressin antagonist; Tolvaptan
Introduction
Heart failure is a growing problem with more than 23 million individuals affected worldwide and over 5.8 million affected in the United States (US) [1]. In 2015, the medical cost related to Congestive Heart Failure (CHF) in US was estimated at 31 billion dollars and is projected to increase 3 fold by 2030 [2]. Hospital readmissions for CHF are a major driver of this expense [3-5]. Hyponatremia is one of the major predictors of hospital readmission in CHF [3,4]. Inadequate treatment of hyponatremia in CHF is seen in 41.9% of patients and is independently associated with about 50% increase in the odds of 30 day unplanned readmission or death [5]. Management of hyponatremia, especially in the setting of acutely decompensated CHF can be challenging. Lack of a consistent approach causes hyponatremia to be inappropriately managed in about 43.6% of patients [6]. Evidence suggests that adequate management of hyponatremia in hospital setting may help decrease CHF related morbidity, mortality, and health care cost [6-10]. This article will review the mechanism of hyponatremia in CHF; its prognostic implications; and the available evidence based management strategies.
Pathophysiology of Hyponatremia in Congestive Heart Failure
Hyponatremia is defined as a serum sodium concentration of < 135 mEq/l. In CHF there is decrease in cardiac output and systemic blood pressure which decreases the perfusion pressure of the carotid sinus baroreceptor and renal afferent arteriole. This leads to release of “hypovolemic hormones” such as renin, angiotensin II, Arginine Vasopressin (AVP) and norepinephrine. These neuro-hormonal changes limit salt and water excretion disproportionately leading to volume overload and dilutional hyponatremia (Figure 1).
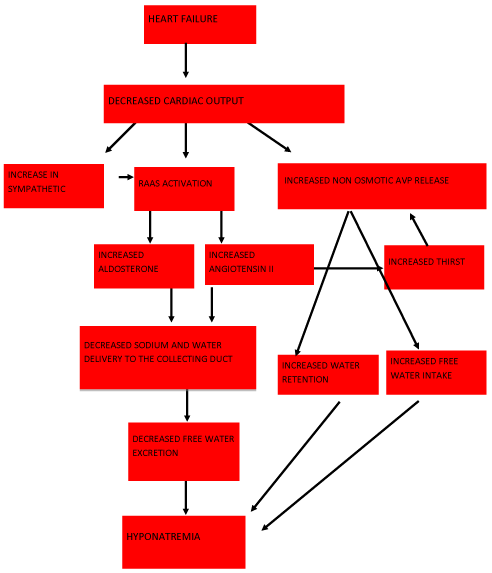
Figure 1: Schematic representation of hyponatremia in CHF.
AVP binds to the Vasopressin-2 (V2) receptor subtype and increases the number of Aquaporin-2 water channels, leading to increased permeability of water in the collecting duct and enhanced free water retention. Angiotensin II and norepinephrine release limit distal sodium and water delivery by lowering Glomerular Filtration Rate (GFR) and by increasing proximal sodium and water reabsorption. Angiotensin II also stimulates the thirst center of the brain promoting increased water intake and further release of AVP [7].
Besides these neuro-hormonal changes, concomitant diuretic use in CHF worsens hyponatremia. As these neuro-hormonal changes are related to the severity of CHF, hyponatremia is often seen in advanced stages of CHF. This confounds the poor prognostic implications of hyponatremia. It is uncertain if hyponatremia is directly associated with increased mortality or if the serum sodium level is a surrogate marker of a more severe underlying disease [8].
Prognostic Value of Hyponatremia in Congestive Heart Failure
Hyponatremia is an independent predictor of increased morbidity and mortality in CHF. The prevalence of hyponatremia in acute heart failure ranges from 8-28% [9,10]. Studies have shown that in patients hospitalized with acute CHF, hyponatremia was independently associated with lower short term and long term survival; longer hospital stay, and increased readmission rates [11,12]. Table 1 summarizes the landmark studies elucidating the prognostic value of hyponatremia in CHF.
Source
Aim
Sample size
Prevalence of hyponatremia
Inclusion criteria
Outcome
ESCAPE
2005 [31]
Association of serum sodium level with 6-month post-discharge all- cause mortality and CHF re-hospitalization
433
23.8%
Heart failure with reduced ejection fraction(HFrEF) NYHA class IV
Hyponatremia was an independent predictor of all-cause mortality (p value = 0.04), CHF re-hospitalization (p value 0.03) and combined death or re-hospitalization (p value = 0.01).
OPTIMIZE-HF
2007 [32]
Relationship between admission serum sodium and clinical outcomes in hospitalized CHF patients.
48,612
19.7%
New-onset or worsening CHF
Hyponatremia was associated with longer length of stay; and greater in-hospital and post-discharge mortality
(p value <0.0001)
Baldasseroni
et al
2011 [33]
Investigate the relation between hyponatremia and mortality
4670
≈10%
Hospitalized due to worsening CHF
Serum Sodium decrease of 1 mEq/L was associated with 10% increase in all cause mortality(p<0.001) and 3% increase in risk for hospitalization( p= 0.005)
Analysis from the Korean Heart Failure (KorHF) registry
2012 [34]
Clinical impact of hyponatremia improvement during hospitalization on post discharge outcome in patients admitted for acute CHF
2888
19.9%
Patients with acute CHF
Persistent hyponatremia is associated with increased incidence of death or re-hospitalization
(HR 1.345, 95% CI 1.075 to 1.683, p=0.010)
Banish etal.
2014 [35]
Prevalence, risk factors, and long-term outcomes of hyponatremia in ambulatory heart failure with preserved ejection fraction (HFpEF) and HFrEF
8862
12.9% in HFpEF and
13.8% in HFrEF
Cohort of veterans with CHF treated in ambulatory clinics
Hyponatremia was a predictor of all-cause mortality in both HFrEF(p <0.001)
and HFpEF(p = 0.004); and predictor of all-cause hospitalization in HFrEF (p <0.001)
but not in HFpEF (p = 0.21)
JCARE-CARD database
2014 [36]
Association of hyponatremia with in-hospital and long-term outcomes
1659
10.6%
Hospitalized due to worsening CHF; mean left ventricular ejection fraction (LVEF) was 42.4%
Hyponatremia was independently associated with in-hospital(p <0.001); and long-term adverse outcomes –all cause death, cardiac death and re-hospitalization due to CHF (p < 0.001)
COAST
2015 [37]
Predictive value of hyponatremia in hospitalized Asian CHF patients
1470
16.8%
= 18 years of age who were hospitalized for CHF with LVEF< 45%
Hyponatremia was an independent predictor of 12-month mortality (p< 0.008) and increased 12-month re-hospitalization rate (p = 0.002)
HARVEST
registry
Lu etal
2016 [38]
Association of the severity of hyponatremia and changes of serum sodium levels with long-term outcome
2556
14.08%
New or exacerbated symptoms and signs of CHF
Independent predictor for all-cause and cardiovascular mortality
( P value = <0.001);
Severe hyponatremia (<125 mEq/L) was associated with worse clinical outcomes
Table 1: Prognostic implications of hyponatremia in CHF.
Pharmacological Management of Hyponatremia in Congestive Heart Failure
Fluid restriction
As described in the introduction, AVP dysregulation and the Renin Angiotensin Aldosterone System (RAAS) are involved in the pathogenesis of hyponatremia in CHF. Fluid restriction is the most inexpensive modality to achieve a negative water balance in these patients. Fluid restriction to less than 1000ml/day in patients with sodium less than 137mg/dl leads to significant improvement in heart failure symptoms [13]. Many patients with heart failure have increased thirst which reduces compliance with strict fluid restriction [14].
AVP antagonist, Tolvaptan is another available treatment modality for hyponatremia in CHF. AVP plays a pivotal role in the decline of sodium concentration in CHF. AVP antagonist, Tolvaptan potentiates excretion of electrolyte free water and increases serum sodium concentration. Tolvaptan has been shown to reduce body weight, increase urine volume, and increase serum sodium concentration in patients with CHF. Meta-analytical studies suggest that tolvaptan is safe, as it does not cause worsening of renal function or hypotension [15-16]. CHF patients on tolvapatan should not be placed on fluid restriction. It is also essential to monitor serum sodium levels every 6-8 hours to avoid rapid correction of hyponatremia [17]. Tolvaptan may confer some mortality benefit in a subgroup of CHF patients with hyponatremia. However, evidence supporting mortality benefit is inconsistent 15 [18].
EVEREST trial, a double blind randomized control trial, studied the use of tolvaptan in patients with worsening CHF. In acute CHF patients with mild to moderate hyponatremia, tolvaptan use showed reversal of hyponatremia. Tolvaptan group had an improvement in serum sodium level of 6.6 mEq/L over 5 days, however, there was no effect on long term mortality or morbidity [19].
Hypertonic saline with Furosemide has been the mainstay of treatment of hyponatremia in patients with CHF with fluid overload. Loop diuretics are preferred because they increase electrolyte-free water clearance [20]. Addition of loop diuretic to Angiotensin Converting Enzyme Inhibitors (ACEI) also helps reverse hyponatremia in CHF patients [21]. This combination of loop diuretic with ACEI has been effective in moderate hyponatremia due to improvement in diluting capacity of the kidneys [22]. Though, hypertonic saline with furosemide is associated with increase in serum sodium levels, but there is only modest improvement in CHF outcomes, such as reduction of length of stay and readmission rates. Thus, this strategy is not as effective as tolvaptan [21,23] and may worsen fluid overload in advanced CHF patients with cardio renal syndrome and diuretic resistance.
Non Pharmacological Management of Hyponatremia in CHF
Ultra-Filtration (UF) is a method of removing fluid from the vasculature without removal of solutes or electrolytes. This method was described as early as 1974 to treat volume overload in CHF patients [24]. Since then several studies have compared UF with pharmacological therapies in CHF. UF improves congestion, lowers right atrial and pulmonary capillary wedge pressure, decreases neurohormone levels, corrects hyponatremia, restores diuresis, and reduces diuretic requirement [23,25]. However, UF has not been shown to improve CHF related outcomes such as hospital re-admission rate or mortality.
In the EUPHORIA trial, Coztanzo et al [26] showed that early UF safely and effectively reduced congestion in Acute Decompensated Heart Failure (ADHF). In this trial UF was initiated within 4.7 ± 3.5 hours of hospitalization in 20 CHF patients with volume overload and diuretic resistance (age 74.5 ± 8.2 years; 75% ischemic disease; ejection fraction 31 ± 15%). UF was initiated at a maximum rate of 500 cc/h and was continued until euvolemia was achieved and symptoms resolved. If systolic BP fell to = 80 mm Hg, UF rate was reduced to 200 cc/h. Re-evaluation was done each hospital day during hospitalization, at 30 days, and at 90 days. Results showed that early UF permitted discharge of 60% of the high risk acutely decompensated CHF patients in = 3 days compared to the ADHERE study where median CHF hospital length of stay was 4.3 days [27]. In spite of the large amount of fluid removed by UF, there was no associated worsening of renal failure, electrolyte abnormalities or symptomatic hypotension. Thus, UF is an effective management strategy for patients with hyponatremia and ADHF.
UNLOAD trial [28] compared UF with standard intravenous (IV) diuretic treatment in ADHF patients. This was a multicenter prospective randomized control trial of 200 ADHF patients (mean age 63 ± 15 years, with 69% men, 71% had LVEF = 40%). The duration and rate (maximum 500 ml/h) of fluid removal by UF were decided by treating physicians. In the UF group, fluid was removed at an average rate of 241 ml/h for 12.3 ± 12 hours. In the standardcare group, average IV daily diuretic dose during the 48 hours after randomization was 181 ± 121 mg. At 48 hours, the UF group lost significantly more weight (5.0 ± 3.1 kg vs. 3.1 ± 3.5 kg; p = 0.001) and fluid (4.6L vs. 3.3 L; p = 0.001) than the diuretic cohort. Dyspnea scores did not differ significantly between the two groups. The CHF readmission rate was significantly lower in the UF group (32% versus 18%; p = 0.037). Neither serum creatinine (baseline 1.5 mg/dl) at hospital discharge nor length of hospital stay differed between the treatment groups. No clinically significant changes in serum blood urea nitrogen, sodium, chloride, and bicarbonate, occurred in either group.
The findings of significant weight loss and fluid removal without change in serum creatinine with UF in EUPHORIA and UNLOAD trials are contrary to the results seen in CARRESS-HF trial. CARESSHF trial [29] was a randomized control trial, that randomly assigned 188 patients with ADHF to receive either IV diuretic therapy (n=94) or UF (n=94). Patients were followed for 60 days. In the patient subset with ADHF and abnormal renal function, UF did not cause fluid or weight loss at 96 hours, but led to an increase in serum creatinine. There was no significant difference between the two groups with respect to mortality and re-hospitalization. At 96 hours, serum sodium level in IV diuretic group increased more than the UF group (0.0 ± 3.6 vs. -2.3 ± 3.5, p < 0.001). These findings illustrate that, despite significant relief of congestion with or without preservation of renal function, UF did not lead to reduction in CHF re-hospitalization rates or mortality.
While UF removes isotonic fluid without exchange in solute or electrolytes, Hemodialysis (HD) involves significant exchange of electrolytes and solutes between dialysate solution and blood across a semi-permeable membrane. Combination of HD and UF is often used in patients with renal failure to correct volume overload and electrolyte abnormalities, with clearance of toxins [30]. Thus UF is an available treatment modality for ADHF patients with hyponatremia; however, its use should be individualized based on the severity of renal dysfunction associated with ADHF.
Given the above study the question that arises is whether UF seems justified due to its high cost and no advantage in comparison to pharmacologic therapy. CARESS-HF study cannot lead us to a definitive conclusion because in CARESS-HF, the duration of UF was longer than other studies which could have resulted in elevated serum creatinine secondary to intravascular volume depletion.
Conclusion
Hyponatremia in CHF is associated with increased morbidity and mortality. Inadequate treatment of hyponatremia in patients admitted for acutely decompensated heart failure is frequent and is associated with poor outcomes. Fluid restriction; Tolvaptan use; hypertonic saline with loop diuretic use; and dialysis are the available treatment modalities which improve morbidity but not mortality. Patients with CHF complicated by hyponatremia should be carefully evaluated in terms of their fluid status, cardiorespiratory status, and renal function prior to initiating any of the above mentioned therapeutic approaches. Treatment of hyponatremia in HF needs to be tailored to the individual patient.
References
- Bui AL, Horwich TB, Fonarow GC. “Epidemiology and risk profile of heart failure”. Nature reviews. Cardiology. 2011; 8: 30-41.
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. “Forecasting the Impact of Heart Failure in the United States: A Policy Statement from the American Heart Association”. Circulation: Heart Failure. 2013
- Jencks SF, Williams MV, Coleman EA. “Rehospitalizations among Patients in the Medicare Fee-for-Service Program”. N Engl J Med. 2009; 360: 1418- 1428.
- Hernandez MB, Schwartz RS, Asher CR, Navas EV, Totfalusi V, Buitrago I, et al. “Predictors of 30-day readmission in patients hospitalized with decompensated heart failure”. Clinical cardiology. 2013; 36: 542-547.
- Kazory A, Donze JD, Beeler PE, Bates DW. “Impact of Hyponatremia Correction on the Risk for 30-Day Readmission and Death in Patients with Congestive Heart Failure”. The American Journal of Medicine. 2010.
- Miller A, Kuehl B, Tennankore K, Soroka S. “Approach to hyponatremia in congestive heart failure: a survey of Canadian specialist physicians and trainees”. Canadian journal of kidney health and disease. 2016; 3: 4.
- Verbrugge FH, Steels P, Grieten L, Nijst P, Tang WH, Mullens W. “Hyponatremia in acute decompensated heart failure: depletion versus dilution”. Journal of the American College of Cardiology. 2015; 5: 480-492.
- Lilly LS, Dzau VJ, Williams GH, Rydstedt L, Hollenberg NK. 1984, “Hyponatremia in congestive heart failure: implications for neuro humoral activation and responses to orthostasis”. The Journal of clinical endocrinology and metabolism. 1984; 59: 924-930.
- Lu DY, Cheng HM, Cheng YL, Hsu PF, Huang WM, Guo CY, et al. “Hyponatremia and Worsening Sodium Levels Are Associated With Long- Term Outcome in Patients Hospitalized for Acute Heart Failure”. Journal of the American Heart Association. 2016; 4.
- Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg, BH, O’Con-nor CM, et al. “Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry”. European heart journal. 2007; 28: 980-988.
- Hamaguchi S, Kinugawa, S, Tsuchihashi-Makaya M, Matsushima S, Sakakibara M, Ishimori N, et al. “Hyponatremia is an independent predictor of adverse clinical outcomes in hospitalized patients due to worsening heart failure”. Journal of cardiology. 2014; 63: 182-188.
- Nodari S, Jao GT, Chiong JR. “Clinical utility of tolvaptan in the management of hyponatremia in heart failure patients”. International Journal of Nephrology and Renovascular Disease. 2010; 3: 51-60.
- Albert NM, Nutter B, Forney J, Slifcak E, Tang WH. “A randomized controlled pilot study of outcomes of strict allowance of fluid therapy in hyponatremic heart failure (SALT-HF)”. Journal of cardiac failure. 2013; 19: 1-9.
- Ghali JK, Tam SW. “The critical link of hypervolemia and hyponatremia in heart failure and the potential role of arginine vasopressin antagonists”. Journal of cardiac failure. 2010; 16: 419-431.
- Alskaf E, Tridente A, Al-Mohammad A. “Tolvaptan for Heart Failure, Systematic Review and Meta-Analysis of Trials”. Journal of cardiovascular pharmacology. 2016.
- Silverstein ME, Ford CA, Lysaght MJ, Henderson LW. “Treatment of severe fluid overload by ultrafiltration”. The New England journal of medicine.1974; 291: 747-751.
- Schrier RW, Sharma S, Shchekochikhin D. 2013, “Hyponatraemia: more than just a marker of disease severity?”. Nature reviews. Nephrology. 2013; 9: 37-50.
- Shanmugam E, Doss CRMP, George M, Jena A, Rajaram M, Ramaraj B, et al. Effect of tolvaptan on acute heart failure with hyponatremia - A randomized, double blind, controlled clinical trial. Indian Heart J. 2016; 68: 15-21.
- Konstam MA, Gheorghiade M, Burnett JC, Grinfeld L, Maggioni AP, Swedberg, K, et al. “Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial”, Jama. 2007; 297: 1319-1331.
- Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. “Risk factors for thiazideinduced hyponatraemia”. QJM: monthly journal of the Association of Physicians. 2003; 96: 911-917.
- Dzau VJ, Hollenberg NK. “Renal response to captopril in severe heart failure: role of furosemide in natriuresis and reversal of hyponatremia”. Annals of Internal Medicine. 1984; 100: 777-782.
- Elisaf M, Theodorou J, Pappas C, Siamopoulos K. “Successful treatment of hyponatremia with angiotensin-converting enzyme inhibitors in patients with congestive heart failure”. Cardiology. 1995; 86: 477-480.
- Agostoni P, Marenzi G, Lauri G, Perego G, Schianni M, Sganzerla P, et al. “Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency: Failure of furosemide to provide the same result”. The American Journal of Medicine.1994; 96: 191- 199.
- Silverstein ME, Ford CA, Lysaght MJ, Henderson LW. “Treatment of severe fluid overload by ultrafiltration”. The New England journal of medicine.1974; 291: 747-751.
- Marenzi G, Grazi S, Giraldi F, Lauri G, Perego G, Guazzi M, et al.1993, “Interrelation of humoral factors, hemodynamics, and fluid and salt metabolism in congestive heart failure: effects of extracorporeal ultrafiltration”. The American Journal of Medicine.1993; 94: 49-56.
- Costanzo MR, Saltzberg M, O’Sullivan J, Sobotka, P. 2005, “Early Ultrafiltration in Patients With Decompensated Heart Failure and Diuretic Resistance”. Journal of the American College of Cardiology. 2005; 46: 2047- 2051.
- Adams KF, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. “Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE)”. American Heart Journal. 2005; 149: 209-216.
- Costanzo MR, Guglin M.E, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, et al. “Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure”. Journal of the American College of Cardiology. 2007; 49: 675-683.
- Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, et al. “Ultrafiltration in decompensated heart failure with cardiorenal syndrome”. The New England journal of medicine. 2012; 367: 2296-2304.
- Kazory A. “Haemodialysis, not ultrafiltration, can correct hyponatraemia in heart failure”. European Journal of Heart Failure. 2010; 12: 208-208.
- Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Pina IL, et al. “Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial”. Archives of Internal Medicine. 2007; 167: 1998-2005.
- Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg, BH, O’Con-nor CM, et al. “Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry”. European heart journal. 2007; 28: 980-988.
- Baldasseroni S, Urso R, Orso F, Bianchini BP, Carbonieri E, Ciro A, et al. “Relation between serum sodium levels and prognosis in outpatients with chronic heart failure: neutral effect of treatment with beta-blockers and angiotensin-converting enzyme inhibitors: data from the Italian Network on Congestive Heart Failure (IN-CHF database)”. Journal of cardiovascular medicine (Hagerstown, Md.). 2011; 12: 723-731.
- Lee SE, Choi DJ, Yoon CH, Oh IY, Jeon ES, Kim JJ, et al.2012, “Improvement of hyponatraemia during hospitalisation for acute heart failure is not associated with improvement of prognosis: an analysis from the Korean Heart Failure (KorHF) registry”. Heart (British Cardiac Society). 2012; 98: 1798-1804.
- Bavishi C, Ather S, Bambhroliya A, Jneid H, Virani SS, Bozkurt B, et al. “Prognostic significance of hyponatremia among ambulatory patients with heart failure and preserved and reduced ejection fractions”. The American Journal of Cardiology. 2014; 113: 1834-1838.
- Hamaguchi S, Kinugawa, S, Tsuchihashi-Makaya M, Matsushima S, Sakakibara M, Ishimori N, et al. “Hyponatremia is an independent predictor of adverse clinical outcomes in hospitalized patients due to worsening heart failure”. Journal of cardiology. 2014; 63: 182-188.
- Yoo BS, Park JJ, Choi DJ, Kang SM, Hwang JJ, Lin SJ, et al. “Prognostic value of hyponatremia in heart failure patients: an analysis of the Clinical Characteristics and Outcomes in the Relation with Serum Sodium Level in Asian Patients Hospitalized for Heart Failure (COAST) study”. The Korean journal of internal medicine. 2015; 4: 460-470.
- Lu DY, Cheng HM, Cheng YL, Hsu PF, Huang WM, Guo CY, et al. “Hyponatremia and Worsening Sodium Levels Are Associated With Long- Term Outcome in Patients Hospitalized for Acute Heart Failure”. Journal of the American Heart Association. 2016; 4.
