Abstract
A 63-year-old male presented with disseminated targetoid erythema and blisters consistent with erythema multiforme on first sight, he was recently treated with allopurinol for primary uric acid nephropathy by his nephrologist. Though his serum creatinine was in the range of stage 3 chronic kidney disease, the man did not show paleness like usual patients within that group, who might suffer from nephrogenic anemia. Paradoxically, the man appeared plethoric and routine blood test found elevated hemoglobin, red blood cell count and mean corpuscular hemoglobin levels. This rare phenomenon lead to the suspicion of polycythemia vera and later confirmed by bone marrow biopsy. Which means the man was not suffering from primary uric acid nephropathy but secondary to polycythemia vera, a disease that increases erythropoiesis and nucleic acid synthesis. Hydroxyurea instead of allopurinol is what he really needs.
Keywords: Polycythemia vera; Allopurinol; Erythema multiforme; Uric acid nephropathy; Chronic kidney disease
Introduction
Erythema Multiforme (EM) is a skin condition that considered to be a hypersensitivity reaction to infections or drugs, it is not an orphan disease, but we encountered a gentleman presented with EM and a series of other co-morbidities, though rare, they together formed a possibly reproducible clinical scenario that might be seen in any health care providing facility, we believe it is worth noticing.
Case Presentation
A 63-year-old male presented with disseminated painful targetoid erythema and blisters consistent with EM on first sight. He was afebrile, and upon questioning he admitted taking allopurinol 200mg daily recently prescribed by his nephrologist for uric acid nephropathy. Except for hyperuricemia and stage 3 Chronic Kidney Disease (CKD) he was believed to be otherwise healthy. But suspiciously, he appeared too “red” for stage 3 CKD, who might develop nephrogenic anemia and appears faint and pale.
On clinical examination, there was disseminated painful swollen targetoid erythema, some with center blistering (Figure 1). The patient appeared plethoric (Figure 2) when compared to a healthy individual (Figure 3).
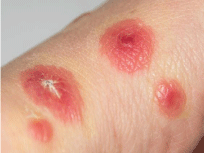
Figure 1: Center blistering.
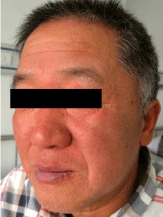
Figure 2: Plethoric.
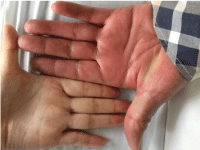
Figure 3: Healthy individual.
After reviewing the patient’s past laboratory tests results, we found some meaningful values as follows: hemoglobin 186 g/L; hematocrit 59.2%; mean corpuscular volume 81fL; serum uric acid 687umol/l; serum creatinine 421umol/l. The simultaneously elevated hemoglobin and Serum Creatinine (SCr) seemed paradoxical, as patients with this level of SCr should be more or less anemic, only Polycythemia Vera (PV) can best explain this paradoxical phenomenon along with elevated serum uric acid. The patient was then sent for hematologist’s consultation and treated with conventional dermatological intervention for allopurinol-associated EM. Later, a bone marrow biopsy confirmed the diagnosis of PV, and the patient was then treated with hydroxyurea 500mg bid for cytoreduction to lower uric acid production instead of allopurinol.
Discussion
PV is a chronic myeloproliferative disease in which the bone marrow makes too many Red Blood Cells (RBC), with or without overproduction of white blood cells and platelets. Excessive nucleic acid metabolism can lead to increased uric acid production, thus, patients with PV are more prone to have gouty arthritis and uric acid nephropathy [1]. Allopurinol is a purine analog and structural isomer of hypoxanthine that can inhibit xanthine oxidase, and subsequently blocking uric acid production [2]. It has been recommended in the use of tumor lysis syndrome prophylaxis [3], although it has gradually been replaced by urate oxidase therapy [4]. Allopurinol alone does not have a therapeutic effect on PV. On the contrary, treatment with allopurinol can increases plasma concentrations of the uric acid precursor’s hypoxanthine and xanthine. Xanthine is poorly soluble, has been demonstrated to cause Glomerular Filtration Rate (GFR) decrease due to precipitation of crystals in renal tubules and stone formation. Kidney injury may thus be exacerbated or triggered by the administration of allopurinol in patients with malignancies [5].
Patient with CKD are frequently anemic known as nephrogenic anemia, it’s a result of insufficient erythropoietin, shortened RBC life span, decreased porphyrin synthesis, or a combination of all these factors. The study by Stauffer et al showed that anemia was twice as prevalent in people with CKD (15.4%) as in the general population (7.6%) in the United States. Although anemia is not as common in earlier stages of chronic kidney disease, the prevalence of anemia increased with stage of CKD, patients with stage 3 disease have a prevalence of concurrent anemia of 17.4%, whereas those with stage 5 disease have a prevalence of concurrent anemia of 53.4% [6].
In the early stage of CKD, there is no obvious increase in SCr. It only increases when the kidney function loses more than 50%, thus, it is considered a less sensitive indicator of kidney function compared to GFR. Secondly, SCr tends to decline with aging, and is considered a poorer predictor of kidney function than GFR. Our patient was only recently diagnosed to have impaired kidney function and a GFR was not rated, we did evaluated patients’ kidney function according to SCr in the past in China, and a stage 3 CKD was diagnosed when the patients’ SCr falls in the range between 176.8-442 μmol/L, the described patient’s SCr was 421 μmol/L when diagnosed. An equation was proposed by Stevens et al that GFR can be estimated with ageadjusted SCr [7]. It’s obvious sex and race should also be taken into account, and unfortunately we don’t have that data in China.
The above case presented with a paradoxical phenomenon of stage 3 CKD sufferer with elevated RBC count, albeit rare, might serve as a pathognomonic series of co-morbidities that indicates specifically PV. PV increases uric acid production, triggers hyperuricemia, gouty arthritis, uric acid nephropathy, and eventually nephrogenic anemia. If the patient was falsely prescribed with allopurinol by a clinician for secondary hyperuricemia without etiological treatment, and he/she unfortunately develop allopurinol-associated drug eruption, then he/ she might finally need a dermatology evaluation.
Allopurinol is associated with several potentially severe Adverse Drug Reactions (ADRs), including Stevens-Johnson syndrome, and toxic epidermal necrolysis. There has been concern that hampered renal excretion of allopurinol predisposes patients with CKD or acute kidney injury to these ADRs, although it is unclear whether these reactions are dose-related. A cohort study of 120 patients, some of whom received allopurinol with dosing adjusted according to their reduced GFR while others received standard dosing, showed no increase in the rate of ADRs in the standard dosing group [8].
The HLA-B*58:01 allele is a genetic marker for allopurinolinduced severe cutaneous adverse reactions, the frequency of the HLA-B*58:01 allele varies between ethnic groups: Han Chinese have HLA-B*58:01 allele frequencies of around 13.99% [9], as compared to Caucasians in Portugal, who have the allele frequencies of 1.96% [10]. The American College of Rheumatology recommends screening for HLA-B*58:01 in high-risk populations (e.g. Koreans with stage 3 or worse CKD and those of Han Chinese and Thai descent), and prescribing patients who are positive for the allele an alternative drug [11]. The Clinical Pharmacogenetics Implementation Consortium guidelines state that allopurinol is contraindicated in known carriers of the HLA-B*58:01 allele [12]. Our patient’s HLA-B*58:01 allele status is unknown, our report here focus more on the importance of recognizing PV induced secondary uric acid nephropathy with “paradoxical hyperhemoglobinemia”, which can be easily identified through routine blood test.
References
- Spivak JL. Polycythemia vera and other myeloproliferative diseases. Longo DL, Fauci AS, Kasper DL, Hauser SL, et al, editors. In: Harrison’s principles of internal medicine. 18th edn. McGraw Hill. 2012; 898-904.
- Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006; 58: 87-114.
- Band PR, Silverberg DS, Henderson JF, Ulan RA, Wensel RH, Banerjee TK, et al. Xanthine nephropathy in a patient with lymphosarcoma treated with allopurinol. N Engl J Med. 1970; 283: 354-357.
- Cairo MS, Coiffier B, Reiter A, Younes A. TLS Expert Panel: Recommendations for the evaluation of risk and prophylaxis of Tumour Lysis Syndrome (TLS) in adults and children with malignant diseases: An expert TLS panel consensus. Br J Haematol. 2010; 149: 578-586.
- Jeha S. Tumor lysis syndrome. Semin Hematol. 2001; 38: 4-8.
- Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014; 9: 84943.
- Stevens LA, Levey AS. Current status and future perspectives for CKD testing. Am J Kidney Dis. 2009; 753: 17-26.
- Vazquez-Mellado J, Morales EM, Pacheco-Tena C, Burgos-Vargas R. Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis. 2001; 60: 981-983.
- Cao ZH, Wei ZY, Zhu QY, Zhang JY, Yang L, Qin SY, et al. HLA-B*58:01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese. Pharmacogenomics. 2012; 13: 1193-1201.
- Gonçalo M, Coutinho I, Teixeira V, Gameiro AR, Brites MM, Nunes R, et al. HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br J Dermatol. 2013; 169: 660-665.
- Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic non pharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012; 64: 1431-1446.
- Hershfield MS, Callaghan JT, Tassaneeyaku W, Mushiroda T, Thorn CF, Klein TE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther. 2013; 93: 153-158.
