Editorial
Ion channels are pore-forming proteins, located in the plasma membrane of almost all living cells. These protein complexes are often highly selectively allowing the flow of particular inorganic ions, primarily sodium (Na+), potassium (K+), calcium (Ca2+) or chloride (Cl-) through aqueous pores across the lipid bilayer. Ion channels play a vital role in physiological processes such as muscle contraction, neuronal signaling, and nutrient transport and therefore human life would not exist without these pore-forming proteins. Likewise, these proteins are functional in the membranes of intracellular organelles such as the endoplasmic reticulum and mitochondria [1,2].
The movements of ions through their pores create an electrical current and these signals permit crucial functions such as the brain to receive and process information, the heart to beat and muscles to work [1]. In other words, they play main roles in very important physiological and pathological processes. Therefore, ion channels serve as a drug target in many important diseases. This manuscript will highlight the therapeutic agents that target ion channels and are currently used in the clinic to treat vital diseases and disorders.
Ion Channels as Drug Target in Cardiovascular System
Cardiovascular diseases are still the number one cause of death globally. Certainly, to maintain normal action potential duration and vascular tone, cardiovascular ion channels are required. Therefore, cardiovascular ion channels represent a critical target in clinic as a therapeutic target to treat important diseases such as arrhythmias, angina and hypertension.
In vascular smooth muscle cells, ion channels play a crucial role in the regulation of vascular tone (contractile activity). Opening of potassium channels causes’ potassium efflux and subsequently, hyperpolarizing the membrane potential which leads to vasodilation. Next, the closer of potassium channels depolarize the cells then activation of voltage gated calcium channels triggers the calcium release from intracellular stores which initiates the contraction [3-6].
Hypertension, which is high blood pressure and a major health problem, can lead to atherosclerosis, heart attack, stroke, enlarged heart and kidney damage. Ion channel agonist or antagonist through theirs vasodilator effect are used in the clinic to treat hypertension. Voltage-gated calcium channels (L-type) are targeted by various drugs (blockers) such as verapamil, amlodipine and nifedipine to treat hypertension [7]. Likewise, potassium channel activator, diazoxide, which target ATP-sensitive potassium (KATP) channels, is used as a vasodilator in the treatment of acute hypertension [1].
Atheromatous plaques gradually narrow the arteries with increasing age which cause obstruction to blood flow and finally symptoms of angina pectoris. Calcium channel blockers (voltagegated, L-type), such as verapamil, amlodipine, and diltiazem are used treat angina [8]. Similarly, nicorandil, which is KATP channel agonist and vasodilator, is used to treat angina [1].
An action potential is a transient, regenerating change in membrane potential that permits a wave of electrical excitation to pass along the plasma membrane of electrically excitable cells. Action potential consists of two major phases; depolarization and repolarization [9]. The heart rhythm abnormality (cardiac arrhythmias) occurs when the electrical impulses that coordinate the heartbeats don’t work appropriately. Ion channels, which are the key player in regulation of generating the action potential and heart rhythm, are targeted to treat cardiac arrhythmias.
Antiarrhythmic drugs are classified by their effects on the cardiomyocyte action potential and are mostly ion channel blockers [10,11]. As an example, these agents target L-type calcium channels (verapamil), voltage-dependent sodium channel (lidocaine) and amiodarone which block the potassium and sodium channels [10,11]. Development of new compounds to treat cardiac arrhythmia is necessary to improve the efficacy and prevent proarrhythmic effects (life-threatening arrhythmias) of the classic antiarrhythmic agents [11,12].
Ion Channels as Drug Target in Neurological Diseases and Disorders
Ion channels play a critical role in the nervous system particularly in neurons such as muscle and excitable neuronal tissue. Various types of channels are involved in neurological functions allowing the brain to receive and process information. These channels act as communication pathways allowing ions to move in and out of the cells through the membrane [13,14].
Plethora of studies report on the importance of ion channels in neurological diseases and disorders. Ion channel regulation, dysfunction or mutation in ion channels (channelopathies)or its regulatory proteins within the nervous system cause numerous neurological and/or neuromuscular diseases and disorders among them multiple sclerosis, amyotrophic lateral sclerosis, Alzheimer’s, Parkinson’s, Huntington’s, epilepsy, pain, depression, anxiety and deafness [13-18]. Hence, neuronal ion channels are considered as potential therapeutic targets to treat neurological diseases and disorders. Currently, ion channels are therapeutically targeted to treat epilepsy and pain.
An estimated 50 million people suffer from epilepsy across the globe with a further 2.4 million diagnosed yearly. A majority of those who suffer from this disorder, 60 percent, have idiopathic epilepsy which has no detectible cause. The remaining suffer from symptomatic epilepsy with a known cause such as a brain damage from prenatal or perinatal injuries including traumatic birth with oxygen loss or low birth weight, a stroke, a brain tumor and certain genetic syndrome [19].
An epileptic seizure takes place when too much electrical signaling occurs in the brain causing uncontrolled, excitatory synaptic transmission. Voltage-Gated Sodium (NaV) ion channels play an essential role in driving the electrical signals in the brain. Therapeutic agents such as phenytoin, carbamazepine and topiramate, which are NaV channel blockers, are used to treat epilepsy [15,20,21]. Also, lignocaine which is a sodium channel blocker is used as local anesthesia [22]. Similarly, gabapentin and ziconotide target the calcium channel (blocker) and are used to treat pain [17,23].
Ion Channels as Drug Target in Diabetes
KATP channels are crucial in the regulation of glucose-induced insulin secretion. In pancreatic β-cells, an increase in ATP/ADP ratio, which is generated by glucose uptake and metabolism, closes the KATP channels to elicit membrane depolarization, calcium influx and a secretion of insulin, the primary hormone of glucose homeostasis [1]. In the pancreatic β-cell (Figure 1), KATP channels are composed of the Kir6.2 pore with the SUR1 regulatory subunit and regulate insulin release. Insulin release is generated by the opening of voltagegated Ca2+ channels and Ca2+ influx. In hyperglycaemia, an increased transport of glucose occurs into the β-cells resulting in an elevated intracellular ATP, promoting closure of the KATP channels and membrane depolarization [1,24]. This KATP channel mechanism can be mimicked by sulphonylurea drugs, for example, glibenclamide, which inhibit the KATP channel directly in the pancreatic β-cells (Figure 1). The inhibition causes cell membrane depolarization, opening of voltage-dependent calcium channels, thus triggering an increase in intracellular calcium into the β-cell stimulating insulin release [1]. Mutations in KATP channels cause physiological dysfunction of this channel leading to pathological consequences such as permanent neonatal diabetes, developmental delay with epilepsy and congenital hyperinsulinism [1,6] (Figure 1).
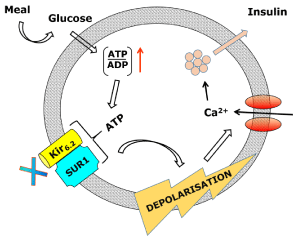
Figure 1: The KATP channel regulation of insulin release in pancreatic β-cells.
When the glucose level increases it causes a rise in the ATP/ADP ratio
and in its turn ATP binds to the KATP channel, which closes the channel.
Depolarization of the membrane then opens the calcium channel which
causes the insulin to release. This drawing illustrates the mimicked pathway
which sulphonylurea drugs by acting to inhibit the KATP channels can
increase insulin secretion, which is used in the treatment of type-2 diabetes.
Summary
Ion channels are membrane proteins which offer pores for the passive diffusion of ions across the biological membranes (the major barrier to ion movement). Consequently, these pore-forming proteins are involved in much important physiological and pathological process (electrical-, chemical signaling, regulation of cytoplasmic ions concentration and cell volume, pH etc.) [1,25,26].
Ion channels dysfunction are associate with many important human diseases and disorders, therefore, these protein complexes serve as drug targets. It is worth mentioning that ion channels also are potential biomarker in cancer (breast, prostate and kidney) or serve as therapeutic target in cardiac fibrosis, cystic fibrosis and platelet [27-32].
Taken together, our understanding of ion channel biology has significantly increased for the past three decades. However, the future direction and the challenge will be to identify small-molecules that are potent, selective, and metabolically stably which can target these unique protein complexes to treat vital diseases and disorders in the clinic.
References
- Rubaiy HN. The therapeutic agents that target ATP-sensitive potassium channels. Acta pharmaceutica. 2016; 66: 23-34.
- Xu H, Martinoia E, Szabo I. Organellar channels and transporters. Cell calcium. 2015; 58: 1-10.
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. The American journal of physiology. 1990; 259: 3-18.
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. The American journal of physiology. 1995; 268: 799-822.
- Hughes AD. Calcium channels in vascular smooth muscle cells. Journal of vascular research. 1995; 32: 353-370.
- Lodwick D, Rainbow RD, Rubaiy HN, Al Johi M, Vuister GW, Norman RI. Sulfonylurea receptors regulate the channel pore in ATP-sensitive potassium channels via an intersubunit salt bridge. The Biochemical journal. 2014; 464: 343-354.
- Michiels CF, Van Hove CE, Martinet W, De Meyer GR, Fransen P. L-type Ca2+ channel blockers inhibit the window contraction of mouse aorta segments with high affinity. European journal of pharmacology. 2014; 738: 170-178.
- Silke B, Goldhammer E, Sharma SK, Verma SP, Taylor SH. An exercise hemodynamic comparison of verapamil, diltiazem, and amlodipine in coronary artery disease. Cardiovascular drugs and therapy/sponsored by the International Society of Cardiovascular Pharmacotherapy. 1990; 4: 457-463.
- Hille B. Ion Channels of Excitable Membranes. Third edn ed: Sunderland, Sinauer Associates. 2001.
- Sanguinetti MC, Bennett PB. Antiarrhythmic drug target choices and screening. Circulation research. 2003; 93: 491-499.
- Ravens U, Poulet C, Wettwer E, Knaut M. Atrial selectivity of antiarrhythmic drugs. The Journal of physiology. 2013; 591: 4087-4097.
- Rubaiy HN, Wardell E, Nylander I, Sylven C. editor Studies on IKr Channel Blockade in Human Cardiomyocytes as a Cause of Arrhythmias. Proceedings of the Physiological Society; University of Oxford, UK: The Physiological Society. 2011.
- Thayer DA, Jan LY. Mechanisms of distribution and targeting of neuronal ion channels. Current opinion in drug discovery & development. 2010; 13: 559-567.
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nature reviews Neuroscience. 2006; 7: 548-562.
- Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. The Lancet Neurology. 2010; 9: 413-424.
- Waszkielewicz AM, Gunia A, Sloczynska K, Marona H. Evaluation of anticonvulsants for possible use in neuropathic pain. Current medicinal chemistry. 2011; 18: 4344-4358.
- Patel R, Dickenson AH. Mechanisms of the gabapentinoids and alpha 2 delta-1 calcium channel subunit in neuropathic pain. Pharmacology research & perspectives. 2016; 4: 00205.
- Galaz P, Barra R, Figueroa H, Mariqueo T. Advances in the pharmacology of lGICs auxiliary subunits. Pharmacological research. 2015; 101: 65-73.
- World Health Organization.
- Black JA, Waxman SG. Phenytoin protects central axons in experimental autoimmune encephalomyelitis. Journal of the neurological sciences. 2008; 274: 57-63.
- Perucca E. A pharmacological and clinical review on topiramate, a new antiepileptic drug. Pharmacological research. 1997; 35: 241-256.
- Brinkrolf P, Hahnenkamp K. Systemic lidocaine in surgical procedures: effects beyond sodium channel blockade. Current opinion in anaesthesiology. 2014; 27: 420-425.
- Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacological reviews. 2015; 67: 821-870.
- Ashcroft SJ, Ashcroft FM. Properties and functions of ATP-sensitive K-channels. Cellular signalling. 1990; 2: 197-214.
- Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nature reviews Drug discovery. 2009; 8: 982-1001.
- Ashcroft FM. From molecule to malady. Nature. 2006; 440: 440-447.
- Leanza L, Manago A, Zoratti M, Gulbins E, Szabo I. Pharmacological targeting of ion channels for cancer therapy: In vivo evidences. Biochimica et biophysica acta. 2016; 1863: 1385-1397.
- Naylor J, Minard A, Gaunt HJ, Amer MS, Wilson LA, Migliore M, et al. Natural and synthetic flavonoid modulation of TRPC5 channels. British journal of pharmacology. 2016; 173: 562-574.
- Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovascular research. 2011; 89: 744-753.
- Rubaiy HN, Linsdell P. Location of a permeant anion binding site in the cystic fibrosis transmembrane conductance regulator chloride channel pore. The journal of physiological sciences : JPS. 2015; 65: 233-241.
- Quon BS, Rowe SM. New and emerging targeted therapies for cystic fibrosis. Bmj. 2016; 352: 859.
- Zhang W, Trebak M. STIM1 and Orai1: novel targets for vascular diseases? Science China Life sciences. 2011; 54: 780-785.
