Introduction
Primary Hyperparathyroidism (PHPT) is the most common cause of hypercalcemia and should be considered in any person with an elevated serum calcium level. The introduction of routine measurement of serum calcium level led to a sharp increase in the incidence of primary hyperparathyroidism in the early 1970s. While population-based studies suggest that the true incidence of PHPT is declining since the 1980, it still remains a relatively common disorder in clinical practice [1,2]. The exact incidence of primary hyperparathyroidism is not known; however, current data suggest a prevalence of 1-4/1000 in the general population [3]. It can occur at any age; it peaks in the seventh decade, 2 to 3 times more commonly seen in women than men, but the incident is similar in men and women younger than 45 years of age [2,4]. The classical manifestations of PHPT include a generalized bone disease, kidney stones, nephrocalcinosis, gastrointestinal, cardiovascular, neuromuscular and neuropsychiatric symptoms. These classic presentations of PHPT are rare today. For this reason the term “asymptomatic primary hyperparathyroidism” is introduced to describe patients who lack obvious signs and symptoms referable to either excess calcium or Parathyroid Hormone (PTH) [5]. Those asymptomatic patients make up to 80% of cases [6]. Nephrolithiasis occurs in 4 to 15% of cases [7]. We present a case of primary hyperparathyroidism presenting with recurrent urinary tract infection.
Case Presentation
A 42-year-old African American woman with no significant medical history presented with compliant of sharp left flank pain of 18 hours duration associated with fever, nausea, and vomiting. She denied dysuria, urgency, frequency, hematuria, vaginal discharge, dyspareunia or history of pelvic inflammatory disease. She had multiple similar episodes in the last six months and was treated with different antibiotics for UTI at other facilities. She had constipation, menstrual irregularity and occasional double vision but denied headache or nipple discharge. She denied family history of kidney stones, thyroid cancer, or hypertension. On physical examination, she had blood pressure of 110/68mmHg, pulse rate of 107 beats per minute, respiratory rate was 18 breaths per minute and temperature of 101.1oF. The oxygen saturation was 100% while breathing on ambient air. She had pale conjunctiva and left costovertebral angle tenderness. She appeared depressed and apathetic. The remainder of the physical examination was unremarkable. Laboratory data showed serum calcium of 11.4mg/ dl and phosphorus of 1.8mg/dl. WBC was elevated at 13,900 per cubic millimeter. Urine analysis showed 10 to 15 RBC per high field microscopy, 5 to 9 WBC per high field microscopy, large leukocyte esterase and positive nitrite. Lactic acid was 2.4 mm/L. CT scan of the abdomen without contrast showed left ureteropelvic junction calculi and perinephric stranding (Figure 1). Blood culture grew Klebsiella pneumoniae. She was treated with levofloxacin. Contrast enhanced CT scan revealed delayed heterogenous left renal enhancement reflecting pyelonephritis with obstructive uropathy (Figure 2). Left nephrostomy tube was placed. On subsequent days, her serum calcium level continued to rise to 14mg/dl. Patient was started on pamidronate and calcitonin.
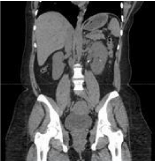
Figure 1: CT abdomen without contrast showed left ureteropelvic junction
calculi and perinephric stranding.
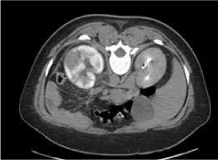
Figure 2: Contrast enhanced CT revealed delayed heterogeneous left renal
enhancement reflecting pyelonephritis with obstructive uropathy.
Further work up of our patient showed elevated PTH at 216pg/ml, low 25-hydroxy vitamin D of 5 ng/ml, and high 1,25-dihydroxyvitamin D of 92pg/ml (Normal 18- 72). The 24 hour urine calcium was 702.6 mg/24 (50 – 300) with the 24 hour urine creatinine clearance of 1.6. Total urine output over the 24 hour was 3925ml and the Urine Creatinine to calcium ratio was 0.002 (< 0.01). The Prolactin was 32.1ng/ml and free T4 of 0.87 nG/dl, T3 Uptake of 44.3%, TSH of 1.46mU/mL, thyroxine total of 8.53 mcg/dl, Total T3 of 79.0nG/dl, and the Serum Cortisol was 22.6 mG/dl. Parathyroid sestamibi scan showed increased tracer accumulation in the left side of the neck consistent with functioning parathyroid adenoma (Figure 3). MRI of the sella was unremarkable. Left parathyroidectomy was performed and intra operative parathyroid hormone level before and immediately after surgery was 550 and 32pg/ml respectively. On the following day, the serum calcium level dropped to 7.5mg/dl suggesting hungry bone syndrome and patient was given calcium replacement. On subsequent days patient felt better, energetic and was discharged with improvement.
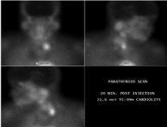
Figure 3: Parathyroid sestamibi scan showing increased tracer accumulation
in the left side of the neck consistent with functioning parathyroid adenoma.
Discussion
The finding of hypercalcemia on routine biochemical test is typically the initial clue to the diagnosis of PHPT. When an elevated total serum calcium concentration is encountered, the clinician should first confirm this finding under conditions that minimize the likelihood of false positive values. About 45% of serum calcium is bound to proteins, mainly albumin, and serum calcium should be corrected for albumin. In hypercalcemic patients who have hypoalbuminemia owing to malnutrition or chronic illness, serum calcium may be falsely normal, but the presence of hypercalcemia will be confirmed by correcting the serum calcium for albumin. Measurement of ionized calcium also allows exclusion of factitious hypercalcemia in selected cases, such as in patients with hyperalbuminemia, thrombocytosis, Waldenstrom’s macroglobulinemia and myeloma; these patients may have elevated levels of total serum calcium, but normal levels of ionized serum calcium. A low urinary calcium to creatinine clearance ratio helps distinguish between primary hyperparathyroidism and familial hypocalciuric hypercalcemia, which can also present with raised or high-normal PTH in the presence of hypercalcemia. In familial hypocalciuric hypercalcemia, the calcium to creatinine clearance ratio is less than 0.01 in about 80% of patients, whereas in primary hyperparathyroidism it is usually greater than 0.01 [8]. The next step in the evaluation of hypercalcemia is measurement of serum PTH. An elevated level or a high normal value of intact PTH in the setting of elevated total or ionized calcium indicates a diagnosis of primary hyperparathyroidism. These laboratory values may also be found in patient who uses lithium or thiazide, familial hypocalciuric hypercalcemia and tertiary hyperparathyroidism associated with end-stage renal disease. A low level of PTH rules out PHPT and raises the possibility of non-PTH mediated hyercalcemia. Investigations to exclude a tumor or occult granulomatous disease are necessary; and initial imaging may include chest radiograph, mammogram, bone scan and ultrasound of the abdomen and pelvis. Serum immunoelectrophoresis is also to be considered. Cancer associated hypercalcemia is often mediated by PTH related peptide which does not cross react with the PTH assay. Other non-PTH mediated causes of hypercalcemia include vitamin D toxicity, adrenal insufficiency, thyrotoxicosis, multiple myeloma and granulomatous diseases. Even though it is rare, primary hyperparathyroidism can also exist concomitantly with a tumor. Vitamin D levels should be measured as these patients have been found to have a higher prevalence of vitamin D inadequacy owing to elevations in PTH, thereby enhancing the conversion of 25-hydroxy vitamin D to 1, 25-dihydroxy vitamin D. Vitamin D inadequacy can be associated with higher PTH level after parathyroidectomy and a higher risk of hungry bone syndrome after surgery [9]. Parathyroid imaging has become a standard preoperative procedure to locate abnormal parathyroid tissue. Parathyroid imaging is not a diagnostic procedure and is not advised unless surgical intervention is planned. Imaging techniques most frequently used are 99mTc-sestamibi scintigraphy, ultrasound, MRI and CT scan [10].
All patients with confirmed symptomatic Primary Hyperparathyroidism (PHPT) should undergo surgical treatment. The threshold value of serum calcium for which surgery is recommended is more than 1mg/dL (>0.25mm/L) above the upper limit of normal. In some patients with asymptomatic disease, surgery is not mandatory. On the other hand, even in these subjects who don’t meet any criteria for parathyroidectomy, surgery is always an option because it is the only definitive therapy for PHPT. The latest guideline recommendation for monitoring those patients who do not undergo parathyroid surgery includes annual serum calcium and creatinine, every 1 to 2 years X-ray or VFA of spine if clinically indicated, and if renal stones are suspected, a 24-h biochemical stone profile, renal imaging by x-ray, ultrasound, or CT scan [11]. Medical therapy is indicated to reduce the level of hypercalcemia in patients who are not eligible for or refuse to undergo parathyroidectomy, pregnant women in their first or third trimester, and to patients who failed surgical treatment [12].
Conclusion
Primary hyperparathyroidism is the most common causes of hypercalemia in the outpatient setting. Most of the patients remain asymptomatic. Surgery is indicated for patients with signs or symptoms of the disease. Our patient presented with nephrolithiasis despite multiple clinic visit for urinary tract infection. This emphasizes the need to seek for secondary causes of recurrent UTI.
Acknowledgement
The authors would like to thank Dr. Tamrat Retta for reviewing the manuscript.
References
- Wermers RA, Khosla S, Atkinson EJ, Hodgson SF, O’Fallon WM, Melton LJ 3rd. The rise and fall of primary hyperparathyroidism: a population-based study in Rochester, Minnesota, 1965- 1992. Ann Intern Med. 1997; 126: 433- 440.
- Wermers RA, Khosla S, Atkinson EJ, Achenbach SJ, Oberg AL, Grant CS, et al. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993- 2001: an update on the changing epidemiology of the disease. J Bone Miner Res. 2006; 21: 171-177.
- Khan AA, Bilezikian JP, Potts JT Jr. The diagnosis and management of asymptomatic primary hyperparathyroidism revisited. J Clin Endocrinol Metab. 2009; 94: 333-334.
- Pallan S, Rahman MO, Khan AA. Diagnosis and management of primary hyperparathyroidism. BMJ. 2012; 19: 344.
- Silverberg SJ, Walker MD, Bilezikian JP. Asymptomatic primary hyperparathyroidism. J Clin Densitom. 2013; 16: 14-21.
- Gasser RW. Clinical aspects of primary hyperparathyroidism: clinical manifestations, diagnosis, and therapy. Wien Med Wochenschr. 2013; 163: 397-402.
- Rejnmark L, Vestergaard P, Mosekilde L. Nephrolithiasis and renal calcifications in primary hyperparathyroidism. J Clin Endocrinol Metab. 2011; 96: 2377-2385.
- Al-Azem H, Khan AA. Primary hyperparathyroidism. CMAJ. 2011; 183: 685- 689.
- Beyer TD, Chen EL, Nilubol N, Prinz RA, Solorzano CC. Short-term outcomes of parathyroidectomy in patients with or without 25-hydroxy vitamin D insufficiency. J Surg Res. 2007; 143: 145-150.
- Khan AA, Hanley DA, Rizzoli R, Bollerslev J, Young JE, Rejnmark L, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int. 2016.
- Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JT Jr. J Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. Clin Endocrinol Metab. 2014 ; 99: 3561-3569.
- Crowley RK, Gittoes NJ. When would I use medical therapies for the treatment of primary hyperparathyroidism? Clin Endocrinol (Oxf). 2013; 79: 770-773.
