Abstract
Objectives: We aimed to explore the potential therapeutic role of interleukin-22 (IL-22) in kidney injury associated with L-arginine-induced severe acute pancreatitis (SAP) and the potential mechanisms.
Methods: SAP was induced by L-arginine intraperitoneal injections in BALB/c mice. In SAP mice, one group received recombinant mouse interleukin 22 (IL-22; 200 ng/dose, 5 times, 24-hr intervals) and the other received phosphate buffer solution at the same times. At 72 hr after the last injection of L-arginine, the severity of acute pancreatitis and associated kidney injury was examined by biochemical and histopathological analyses, and IL-22RA1, Bcl- 2 and Bcl-XL expression in renal tissues of mice was examined by RT-PCR. Signal regulator and activator of transcription 3 (STAT3) and phospho-STAT3 (p-STAT3) proteins were detected by Western Blot.
Results: Administration of L-arginine led to severe pancreatic apoptosis and renal function injury in mice. Pretreatment with IL-22 alleviated the severity of SAP and associated kidney injury by attenuating serum amylase, creatinine and blood urea nitrogen levels and pancreas and kidney pathological injury. IL-22RA1, Bcl-2 and Bcl-XL mRNA levels and p-STAT3 protein level in renal tissues were significantly elevated with IL-22 treatment.
Conclusions: Treatment with exogenous IL-22 reduced the severity of L-arginine–induced SAP and associated kidney injury in mice. IL-22 can up regulate anti-apoptotic genes and activate STAT3 in the kidney of mice.
Keywords: Interleukin-22; Acute pancreatitis; Acute kidney injury; Signal transducers and activators of transcription 3
Abbreviations
IL-22: Interleukin-22; SAP: Severe Acute Pancreatitis; STAT3: Signal Regulator And Activator Of Transcription 3; p-STAT3: Phospho-Signal Regulator And Activator Of Transcription 3; IL- 22RA1: Interleukin-22 Receptor Subunit Alpha 1; Bcl-2: B-Cell Leukemia/Lymphoma-2
Introduction
Kidney injury is a common serious complication of Severe Acute Pancreatitis (SAP) characterized by the development of systemic inflammatory and multiple organ failure syndromes, second only to respiratory dysfunction [1]. Despite recent progress in renal replacement therapy and critical care medicine, kidney injury is still associated with high morbidity and mortality in patients with acute pancreatitis [2]. Thus, studying the pathogenesis and searching for novel therapies are urgently needed.
Apoptotic cell death may play a considerable role in affecting acute pancreatitis-induced kidney injury; control of apoptosis is a potent strategy for improving the clinical outcome in SAP [3,4]. Interleukin-22 (IL-22) may have a protective role in pancreatitisinduced AKI.
IL-22 is a member of the IL-10 cytokine family with pronounced tissue-protective properties; it recently became a major focus of cytokine biology and related translational research [5]. It plays an important role in the proliferation and survival of cells by predominantly activating Signal Transducer And Transcription Factor 3 (STAT3) and up-regulating anti-apoptotic genes including Bcl-2 and Bcl-XL [6,7]. The functions of IL-22 are mediated by binding to IL-10 receptor (IL-10R2) and IL-22 receptor subunit alpha 1 (IL-22RA1). IL-10R2 is ubiquitously expressed in many types of cells, whereas IL-22RA1 is mainly expressed by epithelial cells in various tissues, with physiologically high levels in the skin, the colonic mucosa and pancreas, followed by the liver, lung and kidney [8-11]. In animal experiments, IL-22 treatment ameliorated renal ischemia– reperfusion injury and acute pancreatitis [12,13]. However, whether IL-22 also has a protective effect on acute pancreatitis-associated AKI remains unknown.
In the present study, we examined the effect of IL-22 on pancreatitis-associated AKI in mice and found IL-22 effective in preventing acute pancreatitis progression and preserving renal function, which may be associated with the STAT3 signal pathway.
Materials and Methods
Animals
Seven to eight-week-old BABL/c male mice (18-22g) were purchased from the Experimental Animal Center of Shandong University (Shandong, China). Mice were kept under standardized conditions with a 12-hr light/dark cycle and had free access to a standard diet and water. Animals were allowed to acclimatize for 1 week before experiments. All animal experiments were performed in accordance with the guidelines of the Shandong University Institutional Animal Care and Use Committee.
Experimental design
Animals were divided randomly into 4 groups (n=12 each) for treatment: normal control, 2 intraperitoneal saline injections; Severe Acute Pancreatitis (SAP), 2 intraperitoneal injections of 20% L-arginine (Sigma, USA; 4g/kg body weight, interval of 1hr); IL-22, daily intravenous injection of recombinant mouse IL-22 (Miltenyi, Germany) 200 ng/day for 5 days and on day 3 intraperitoneal injections of 20% L-arginine; and PBS control, 2 intraperitoneal L-arginine injections as for SAP group and Phosphate Buffered Saline (PBS) injections as for IL-22 group. The experimental schedule is shown in Figure 1. The mortality rates of each group were calculated at 72hr after the first injection of L-arginine.
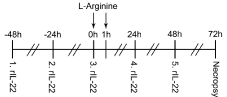
Figure 1: The procedure of interleukin 22 (IL-22) and L-arginine administration
to mice.
Isolation of organs and histopathology
All animals were anesthetized with ether and killed by exsanguination; blood was collected for serum analysis 72hr after injection of L-arginine. Serum was stored at -80°C before measurement. The pancreas and right kidney were harvested and immediately fixed for 24hr in 10% formalin solution and embedded in paraffin. The remaining kidney was stored at -80°C. Fixed tissues were sectioned at 4μm and stained with Hematoxylin and Eosin (HE). Pathology sections were observed by light microscopy. The histologic score and grading were based on edema, inflammation, hemorrhage, and parenchymal necrosis [14]. The severity of kidney injury was scored blindly as described [15].
Serum biochemical analysis
Blood was collected by removing eyeballs and centrifuged to separate serum. Serum amylase, Creatinine (Cr), and Blood Urea Nitrogen (BUN) were measured by use of an Olympus AU5400 automatic biochemical analyzer (Olympus Corp., Japan).
Western blot analysis
Protein was extracted by homogenizing half of one kidney in RIPA Lysis Buffer. Protein samples (80μg) were separated on 8% SDS-PAGE. After electrophoresis, the proteins were transferred by electroblotting to PVDF membrane filters (Millipore, USA), which were washed in Tris-buffered saline containing 0.1% Tween 20 (TBS-T), blocked with 5% nonfat dry milk in TBS-T for 1hr at room temperature, washed again in TBS-T and incubated at 4°C overnight with the primary rabbit polyclonal antibodies anti-STAT3 (1:2000) and anti-phospho-STAT3Tyr705 (1:2000) (Cell Signaling, MA), then washed 3 times in TBS-T and incubated with a horseradish peroxidase labeled secondary antibody for 1 hr at room temperature. Protein bands were visualized by using chemiluminescence reagent (ECL, Millipore Biotechnology, USA). Image J was used for analysis and β-actin was an internal control.
Real-time PCR
Total RNA was purified from renal tissues by using Trizol reagent (TaKaRa Bio, Osaka, Japan) according to the manufacturer’s instructions and cDNA was synthesized by using the PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa Bio, Osaka, Japan), then mixed with SYBR Green and specific primer sequences. The sequences of the primers used are given in Table 1. RT-PCR involved the LightCycler 480 Real-Time PCR System (Roche, Sweden) with cycle conditions 2min at 95°c, followed by 40 cycles of 10s at 95°c (denaturation), 30s at 60°c (annealing), and 30s at 72°c (extension). Raw data were standardized to GAPDH level, and relative amounts were calculated by the 2-??CT method.
Gene Name
Forward Primer (5´- 3')
Reverse Primer (5´- 3')
Bcl-2
TGAAGCGGTCCGGTGGATA
CAGCATTTGCAGAAGTCCTGTGA
Bcl-xL
GAGGCAGGCGATGAGTTG
ACGATGCGACCCCAGTTT
IL-22RA1
TCTGGGCTACAAATACATCACCAAG
GGCCACTGAGGTCCAAGACA
GAPDH
AAATGGTGAAGGTCGGTGTGAAC´
CAACAATCTCCACTTTGCCACTG
Table 1: Primers for real-time PCR.
Statistical analysis
Data were analyzed by using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) and presented as mean ± SD. Differences were compared by Student’s t-test and Fisher’s exact test. A p value less than or equal to 0.05 was considered statistical significant.
Results
IL-22 Treatment reduces levels of serum amylase and preserves renal function
To check whether induction of pancreatitis and associated kidney injury by L-arginine was successful, we evaluated amylase serum levels and main serum factors of renal function (Cr and BUN). After injection of L-arginine, level of serum amylase was significantly increased (Figure 2A). Furthermore, Cr and BUN levels were higher in mice with than without SAP (Figure 2B, 2C). These indexes did not differ between PBS and SAP groups. However, treatment with IL-22 attenuated the increase in these levels relative to PBS treatment (Figure 2).

Figure 2: Serum biochemical determination of amylase, creatinine and blood
urea nitrogen (BUN) with mouse groups normal, Severe Acute Pancreatitis
(SAP), Phosphate-Buffered Saline (PBS) and IL-22 (see Methods). Data are
mean ± SD. Statistical comparisons were performed on SAP group and IL-
22 group. Asterisks indicate significant differences from control value. (*p <
0.05).
Histopathological lesions of pancreas and kidney mitigated by IL-22 treatment
HE-stained histological sections showed no pancreas or kidney damage in normal mice (Figure 3A, 3E). In contrast, severe pancreas damage was detected in SAP and PBS mice (Figure 3B, 3C), characterized by extensive acinar cell necrosis, interstitial edema and diffusive infiltration of inflammatory cells. The pancreas damage was less pronounced for mice with SAP and IL-22 treatment (Figure 3D) than PBS treatment (Figure 3C). SAP and PBS groups showed renal tubular-dilatation interstitial edema (Figure 3F, 3G). Compared with PBS mice, SAP mice with IL-22 treatment showed no pathological damage (Figure 3H). In conclusion, the IL-22 therapy significantly reduced the extent and severity of the histological signs of pancreatic and renal injury in SAP mice.
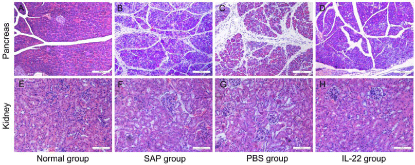
Figure 3: Histopathologic characteristics of pancreatic and renal tissue
in different groups (HE staining). Image A,B,C and D were pancreatic
pathological section (Scale bar: 100 μm); image E,F,G and H were renal
pathological section (Scale bar: 50 μm).
Increased expression of IL-22RA1, Bcl-2 and Bcl-XL genes in kidneys of mice with IL-22 treatment
The above data suggested that IL-22 treatment ameliorated SAPinduced kidney injury. To investigate the mechanisms by which IL-22 attenuates kidney injury in mice with acute pancreatitis, we examined the mRNA expression of IL-22RA1, Bcl-2 and Bcl-XL in renal tissues. The expression of IL-22RA1 was higher with IL-22 than control treatment (Figure 4A). Similarly, the expression of Bcl-2 and Bcl- XL was significantly increased in renal tissues with IL-22 treatment (Figure 4B, 4C). The PBS and SAP groups did not differ in expression of these genes.
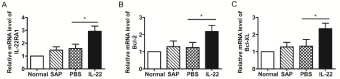
Figure 4: Real-time PCR analyses of mRNA levels of IL-22RA1 (A), Bcl-2
(B) and Bcl-XL (C) in renal tissues of mice. Data are mean ± SD. (*p < 0.05).
IL-22 treatment induces STAT3 phosphorylation after acute pancreatitis
We detected the protein expression of STAT3 and activated phospho-STAT3 (p-STAT3) in renal tissues by western blot analysis. The expression of p-STAT3 was significantly greater with IL-22 than PBS treatment, with no difference in STAT3 protein level between the two treatments (Figure 5A). Accordingly, the ratio of p-STAT3 to STAT3 protein was higher with IL-22 than PBS treatment (Figure 5B).
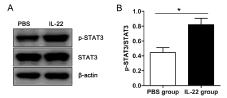
Figure 5: IL-22 treatment induces phosphorylation of STAT3 protein in the
kidney. (A) Western blot analysis of p-STAT3 and STAT3 protein in renal
tissues of SAP mice treated with PBS or IL-22 and (B) ratio of pSTAT3 to
STAT3 protein with IL-22 treatment. (*p < 0.05).
At 72hr after the first L-arginine injection, the mortality rates for the PBS and IL-22 groups were 41.7% and 9.3%, respectively, with no statistically difference between PBS and SAP groups (Fisher exact probability test, p = 0.077, as shown in Table 2).
Cases (n)
survival rate (%)
Mortality rate (%)
IL-22 group
12
91.7 (n=11)
8.3 (n=1)
PBS group
12
58.3 (n=7)
41.7 (n=5)
Fisher exact probability test, p = 0.077.
Table 2: The mortality of mice at 72hr after L-arginine injection.
Discussion
Numerous pre-clinical studies have been conducted in recent years to explore the therapeutic potential of recombinant IL-22 for treating multi-organ diseases associated with epithelial injury. Acute pancreatitis patients show high plasma levels of IL-22, regardless the severity of the disease [16]. Administration of IL-22 reduced acute pancreatitis in experimental animals [17]. However, the effect of exogenous IL-22 for acute pancreatitis-associated kidney injury has been little investigated, with no research about its functional mechanism. Our data showed that exogenous recombinant IL- 22 preserved renal function in mice with SAP and increased the expression of anti-apoptotic genes in the kidney of such mice. IL- 22 may have therapeutic potential for treating acute pancreatitisassociated kidney injury.
Acute pancreatitis models in BALB/c mice are induced by intraperitoneal injections of L-arginine. This animal model is noninvasive, cheap and easy to induce [18,19]. Signs of mild acute tubular necrosis in the kidney can be observed [20]. Furthermore, L-arginine, the nitric oxide donor, has protective effects on renal ischemia–reperfusion injury [21]. L-arginine does not cause direct harm to the kidney, so kidney injury in this model was induced by acute pancreatitis. This laid a feasible foundation for the subsequent experiments.
To investigate the effect of exogenous recombinant IL-22 on the kidney, we detected the expression of IL-22RA1, the epithelial IL-22 receptor, which has a remarkably tissue-specific distribution. IL-22 responsiveness is determined by the expression of IL-22RA1 [6]. We found increased IL-22RA1 expression in renal tissues from mice with IL-22 treatment. This experimental result agreed with previous findings of IL-22RA1 expressed in the renal proximal-tubular epithelial-cell brush border, the most sensitive to acute kidney injury [12]. The result provides a basis for further exploring the protective effect of exogenous IL-22 for the development of acute pancreatitis.
Renal function injury in our mice was alleviated by detecting Cr and BUN level with IL-22 and PBS treatment. IL-22 treatment conferred little renal tubular dilatation in kidneys, whereas animals injected with only L-arginine showed serious histopathological injury in the pancreas and kidney. Because of the massive necrosis, the pancreas size was significantly lower with SAP induction than with IL-22 treatment. IL-22 treatment reduced the extensive pathological injury in pancreatic tissues and the level of serum amylase. These findings suggest that exogenous IL-22 treatment may help ameliorate kidney injury and pancreatitis in L-arginine–induced SAP.
We detected Bcl-2 and Bcl-XL mRNA in the normal mouse kidney and levels were slightly up-regulated in the mouse models of acute pancreatitis. IL-22 administration greatly increased the mRNA expression of Bcl-2 and of Bcl-XL in renal tissues as compared with PBS treatment. Furthermore, exogenous IL-22 treatment activated STAT3 in kidneys and increased the survival rate of mice with SAP. The increase in Bcl-2 and Bcl-XL protein level was associated with increased mRNA expression, so a likely mechanism of increased Bcl- 2 and Bcl-XL mRNA level is transcriptional up-regulation.
Bcl-2 and Bcl-XL are anti-apoptosis proteins of the Bcl-2 family, the main regulators of the intrinsic apoptotic pathway [22]. Upregulation of the pro-survival proteins Bcl-2 and Bcl-XL is a key protective mechanism against necrosis in pancreatitis [23]. STAT3 is involved in phosphatidic acid-induced Bcl-2 expression in HeLa cells [24]. In addition, IL-22 treatment may ameliorate renal ischemia-reperfusion injury by activating STAT3 and Akt signaling pathways. Also, STAT3 is a major downstream signal of IL-22 [12]. STAT3 may mediate the IL-22 hepatoprotective functions by upregulating the expression of anti-apoptotic genes such as Bcl-2 and Bcl-XL [25,26]. We found that recombination mouse IL-22 treatment activated STAT3 and induced a high expression of Bcl-2 and Bcl-XL in renal tissues. Important downstream factors may be involved in the protective role of IL-22 in the kidney, thereby alleviating SAPassociated kidney injury.
Conclusion
IL-22 treatment can prevent L-arginine-induced acute pancreatitis from becoming serious and ameliorate kidney injury in mice. Our findings also suggest that IL-22 activates the STAT3 signal pathway, which can suppress apoptosis [27]. The anti-apoptotic actions of STAT3 are most likely mediated by induction of downstream genes, including Bcl-2 and Bcl-XL. IL-22 might become a novel therapy for severe acute pancreatitis and its severe complications.
References
- Wang M, Lei R. Organ Dysfunction in the Course of Severe Acute Pancreatitis. Pancreas. 2016; 45: 5-7.
- Li H, Qian Z, Liu Z, Liu X, Han X, Kang H. Risk factors and outcome of acute renal failure in patients with severe acute pancreatitis. J Crit Care. 2010; 25: 225-229.
- Takeyama Y. Significance of apoptotic cell death in systemic complications with severe acute pancreatitis. J Gastroenterol. 2005; 40: 1-10.
- Wan L, Bellomo R, Di Giantomasso D, Ronco C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care. 2003; 9: 496-502.
- Muhl H, Scheiermann P, Bachmann M, Hardle L, Heinrichs A, Pfeilschifter J. IL-22 in tissue-protective therapy. Br J Pharmacol. 2013; 169: 761-771.
- Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010; 32: 17-31.
- Nikoopour E, Bellemore SM, Singh B. IL-22, cell regeneration and autoimmunity. Cytokine. 2015; 74: 35-42.
- Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010; 21: 365-379.
- Kotenko SV, Izotova LS, Mirochnitchenko OV, et al. Identification of the functional Interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL- 10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001; 276: 2725-2732.
- Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004; 4: 669-677.
- Tachiiri A, Imamura R, Wang Y, Fukui M, Umemura M, Suda T. Genomic structure and inducible expression of the IL-22 receptor alpha chain in mice. Genes Immun. 2003; 4: 153-159.
- Xu MJ, Feng D, Wang H, Guan Y, Yan X, Gao B. IL-22 ameliorates renal ischemia-reperfusion injury by targeting proximal tubule epithelium. J Am Soc Nephrol. 2014; 25: 967-977.
- Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012; 143: 1670-1680.
- Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005; 115: 3007- 3014.
- Markakis C, Tsaroucha A, Papalois AE, Maria L, Eleftherios S, Christina T, et al. The Role of Eugenol in the Prevention of Acute Pancreatitis-Induced Acute Kidney Injury: Experimental Study. HPB Surg. 2016; 2016: 3203147.
- Vasseur P, Devaure I, Sellier J, Adriana D, Chagneau-Derrodea C, Florian C, et al. High plasma levels of the pro-inflammatory cytokine IL-22 and the antiinflammatory cytokines IL-10 and IL-1ra in acute pancreatitis. Pancreatology. 2014; 14: 465-469.
- Feng D, Park O, Radaeva S, Wang H, Yin S, Kong X, et al. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci. 2012; 8: 249-257.
- Hegyi P, Rakonczay Z, Sari R, et al. L-arginine-induced experimental pancreatitis. World J Gastroenterol. 2004; 10: 2003-2009.
- Kui B, Balla Z, Vegh ET, et al. Recent advances in the investigation of pancreatic inflammation induced by large doses of basic amino acids in rodents. Lab Invest. 2014; 94: 138-149.
- Rakonczay Z, Hegyi P, Dosa S, Ivanyi B, Jarmay K, Biczo G, et al. A new severe acute necrotizing pancreatitis model induced by L-ornithine in rats. Crit Care Med. 2008; 36: 2117-2127.
- Mohamed AdE, Lasheen NN. Comparative study on the protective role of vitamin C and L-arginine in experimental renal ischemia reperfusion in adult rats. Int J Physiol Pathophysiol Pharmacol. 2014; 6: 153-165.
- Loncarevic-Vasiljkovic N, Milanovic D, Pesic V, et al. Dietary restriction suppresses apoptotic cell death, promotes Bcl-2 and Bcl-xl mRNA expression and increases the Bcl-2/Bax protein ratio in the rat cortex after cortical injury. Neurochem Int. 2016.
- Sung KF, Odinokova IV, Mareninova OA, et al. Prosurvival Bcl-2 proteins stabilize pancreatic mitochondria and protect against necrosis in experimental pancreatitis. Exp Cell Res. 2009; 315: 1975-1989.
- Choi HJ, Lee JH, Park SY, Cho JH, Han JS. STAT3 is involved in phosphatidic acid-induced Bcl-2 expression in HeLa cells. Exp Mol Med. 2009; 41: 94-101.
- Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010; 52: 1291-1300.
- Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004; 39: 1332-1342.
- Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009; 1171: 59-76.
