Abstract
On the occasion of a 50-year-old Caucasian female with a history of Chronic/Recurrent Tonsillitis (CRT) accompanied by Palindromic Rheumatism (PR), who presented with ongoing high fever with rigors and a sore throat for the past week, and was finally diagnosed with Necrobacillosis, we conducted an exhaustive, inter-specialties literature review, focused on pathogenesis and autoimmunity with an integral approach. The diagnosis of palindromic rheumatism occurring simultaneously with, or shortly after CRT episodes, was based on the clinical picture of her arthralgias/mild arthritis self-restricted flares, in combination with positive serum RF and anti-CCP auto-antibodies, which were also detected during hospitalization, in addition to anti-SMA/anti-f actin. It is likely that, palindromic rheumatism with auto-antibody production could be a potential manifestation of the wide spectrum of Fusobacterium CRT or better, both to be an integrated disease entity. Furthermore, we review mechanisms of autoimmunity induced by other tonsillar, oral infections, or even periodontitis, comprising mostly Fusobacterium nucleatum. Terms such as reactive arthritis, focal tonsillar infection and rheumatoid arthritis are reviewed in the context of the existence of a common antigen, or that of molecular mimicry between synovial antigen and some kind of bacterial antigen that induces chronic tonsillitis, mostly focused on Fn CRT.
Keywords: Fusobacterium necrophorum; Fusobacterium nucleatum; Necrobacillosis; Auto-immunity; Palindromic rheumatism; Reactive arthritis; Tonsillar focal infection; Periodontitis
Introduction
Despite its rarity, Fusobacterium Necrophorum (Fn) is unique for causing clinically distinctive, severe septicemic infections variously known as Necrobacillosis, postanginal sepsis, or Lemierre’s Syndrome (LS) [1]. We have sought to shed light on the pathogenesis of Chronic/Recurrent Fn Tonsillitis (CRT) and subsequent induction of autoimmunity, as well as on infections caused by Fn and Fusobacterium nucleatum (Fnuc) which have been connected with rheumatic diseases such as palindromic Rheumatism (PR), reactive arthritis, and tonsillar focal infection. The issue of including Fusobacterium species in Guidelines of acute tonsillitis is also been noted.
Case Presentation
Necrobacillosis case
A 50-year-old Caucasian female presented with a week history of sore throat, high fever with rigors, and mild swelling with tenderness along the right sternocleidomastoid muscle. She was treated with clarithromycin and then ciprofloxacin without response. Medical history, besides mood disorders and smoking, was important for CRT attacks with high fever and rigors, accompanied by arthralgias/ mild arthritis that initiated after suffering serologically proven mononucleosis, and were self-restricted. In accordance with positive detection of serum auto-antibodies, she was diagnosed with PR. A month before admission she also reported laborious dental suffixes works.
Clinical examination was notable for pharyngeal erythema with tonsillar exudates, cervical lymphadenopathy, and mild, fine crackles of the right pleural-basis on auscultation. Chest radiology revealed a 3cm coin shading on the right pleural-basis (Figure 1a). Laboratory values were important for highly increased White Blood Cell (WBC) count (25,500/μL, 80% neutrocytes), elevated serum ALT, ALP, ?-GT, and significantly increased inflammatory markers [CRP (208mg/L), ESR (122mm/hr), PCT (2.56pg/mL)]. Blood and urine cultures were drawn and she was admitted to the hospital.
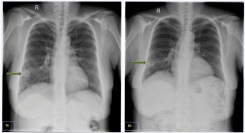
Figure 1:
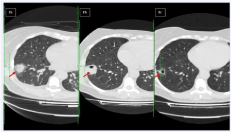
Figure 2:
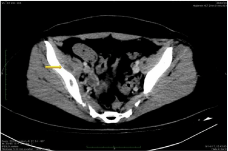
Figure 3:
Empiric medication wasn’t commenced in order blood cultures to be obtained. On day 2, an emerging pelvic pain restricted abduction of her right hip, clinically imitating sacroilitis, and made her bedridden requiring high doses of opioids to keep her comfortable. Later, she developed acute dyspnea with hypoxia requiring high oxygen mixtures supplementation. She also started having a cough productive of bloody sputum. An urgent whole body CT scan revealed swollen cervical lymph nodes with intense hyper perfusion, indicative of possible lymphoma; three unilateral predominantly pleural-based nodular opacities with a surrounding ground glass halo; and diffuse bilateral alveolar infiltrates, (Figure 2a). Patient was commenced on empiric antibiotics. A transthoracic heart ultrasound excluded valvular vegetations. Laboratory reported three anaerobic blood cultures drawn at different times, that grew gram-negative rods subsequently identified as Fn, and treatment was modified to meropenem and metronidazole iv. The same bacterium was identified in sputum cultures.
Despite the relatively prompt stabilization of her respiratory function, the patient continued to spike fevers daily on appropriate antibiotics, while WBC and inflammatory markers during the whole first week sustained in high values. A pelvic MRI remarkably depicted multiple cystic formations (8cm x 3.2cm) with diaphragms within the right iliac muscle, with fat imprecision and a small amount of liquid, consistent with abscess formation CT image (Figure 3). A followup CT showed that the diffuse bilateral alveolar infiltrates, probably owned to ARDS had vanished, while the nodular opacities appeared with clear cavitations (Figure 2b).
After two weeks patient’s general condition had significantly improved, pelvic pain subsided and she started walking in the ward. A new imaging, revealed a significant reduction in pelvic abscess’ size (2.2cm). She was discharged with amoxicillin-clavulanate and metronidazole per os to complete a total treatment of 8 weeks. A follow-up chest CT showed that the largest opacity was significantly reduced in size, while the other two had disappeared (Figure 2c). She recovered without recurrences and with a complete restoration of her walking ability.
Discussion
Fusobacterium are obligate anaerobic gram-negative bacilli which inhabit the oral, gastrointestinal, upper respiratory and genitourinary tract as part of normal flora [2]. Infection occurs through disruption of mucosal surfaces from prior infection, trauma, or tumor [2]. Incriminated prior infections include EBV, CMV, influenza, GAS and Mycoplasma pneumonia [3]. Transient infection-associated mucosal or systemic immunosuppression may contribute to the pathogenesis [3]. Fn may be acquired by close human-to-human contact [4]. Fnpositive individuals are significantly more likely to have had one or more lip-to-lip kissing contacts in the previous four weeks than those who are negative [5].
Clinical entities of Fn infections comprise necrobacillosis of the head-and-neck and outside. Most LS cases are young, healthy males who develop severe septic infections. Others include deep neck space infection, peritonsillar abscess, otogenic, odontogenic, sinusitis, and intracranial complications [1]. Metastatic complications of Fusobacterium Bacteremia (FB) include pleuropulmonary, bone, joint, muscle, and skin lesions, intra-abdominal sepsis, and endocarditis/pericarditis [1]. Disseminated non-head-and-neck Fn infections occur in elderly men, who frequently have cancers related to the primary infectious focus [6]. Predisposing factors comprising cardiovascular/pulmonary disease, renal insufficiency, haematological malignancies, hepatitis and alcohol abuse, carry a worse prognosis [7].
Tonsils were the primary source of infection in our case. Jugular thrombophlebitis indicated by patient’s sternocleidomastoid tenderness wasn’t demonstrated by radiology. However, small pharyngeal veins thrombosis could have occurred. In that case, the pulmonary condensations may have originated from embolic spread. Alternatively, they could be due to direct aspiration of infectious material [7]. The extended iliac muscle abscess on the other hand possibly resulted from blood dissemination of Fn with subsequent hematogenous seeding, initially causing myositis, and directly resulting into abscess formation. The formation of an abscess in FB may protect the relatively fastidious obligate anaerobe from the oxidative environment of the host [7]. Fn-caused iliac muscle abscess is never reported in the literature.
The explanation for Necrobacillosis slow clinical and laboratory response to treatment is that antibiotics are slow to sterilize necrotic abscess, which can be the cause of ongoing fevers [8]. Others attribute that phenomenon to the endovascular nature of Fusobacterium infection [9]. Hyperbilirubinaemia in FB is attributed to a direct effect of Fn haemolytic endotoxins activity [6,9]. In our case slightly increased ALT and biliary enzymes were measured, that followed a slow-restoration pattern. This could conceivably be attributed to liver micro-abscesses that couldn’t be traced by radiology. In addition, serum screening for collagen auto-antibodies revealed positive RF (measured twice), ASMA (IFA), anti-F Actin (ELISA) and anti- CCP (ELISA). Serum detection of infectious agents was notable for positive anti-HAV IgM, a finding incompatible with patient’s clinical picture, as well as weakly positive IgM antibodies and IgG (1:160) to Mycoplasma pneumoniae. Due to RF positivity, both results were considered false-positive. However, a mycoplasmal Upper Respiratory Tract (URT) infection could have proceeded, diverting an Fn carriage to Necrobacillosis [3].
Patient had a 4-year history of sudden febrile angina episodes that started after serologically proven mononucleosis. Attacks were accompanied, or followed by arthralgias/arthritis mostly of the hand joints and lower limps. Episodes lasted for a few days, were selfrestricted without antibiotics, and articular symptoms responded to magnesium preparations. Mg++ may block the N-methyl-D-aspartate (NMDA) receptors via CluN2 subtypes that facilitate the peripheral sensory transmission of pain signals. Activation of NMDA leads to Ca++ entry into the cell triggering protein kinase C activation, NO and superoxide production [10]. NO and superoxide can form peroxynitrite that produces the pain. All those events associated with persistent pain are blocked by Mg++.
Mounting evidence are strongly suggestive that Fn has a causative role in CRT [1,3,8]. Fn-associated peritonsillar abscess tends to arise in tonsils affected by recurrent infection [1]. Jensen et al., detected Fn DNA in 48% of throat swabs in patients with tonsillitis in significantly higher loads than in 21% of the positive healthy controls [11]. They confirmed that Fn probably resides in small numbers in the crypts of the tonsils in healthy humans and can cause acute or chronic tonsillitis. Thus, if swabs are taken only from the surface of the tonsils, Fn might be falsely negative. The latter, could explain the inconsistent Fn carriage rates reported in different studies. Jensen et al., conducted a molecular mapping of the tonsillar crypt microbiota to species level [12]. A core microbiome of eleven predominant members is present in the crypts, regardless of age and health statue of the tonsils, including Fusobacterium species. Phylogenetic analysis revealed that all samples were dominated by Fusobacterium nucleatum (Fnuc), except for CRT cases who had Fn in substantial amounts in all samples. Microbiota of the URT was found in only small proportions in the tonsillar crypts, thus constituting them a unique habitat, more reminiscent of the periodontal microbiota [12]. Dapefrid et al., determined that Fn was the most prevalent in tonsils both at the surface and in the core from CRT patients subjected to tonsillectomy [13]. In another cohort, Fn was found in 28, 30 and 16% at inclusion, surgery, and follow-up respectively. Authors, attributed the lack of correlation between symptomatology and the presence of Fn in the 16% of postoperative swabs, to the assumption that bacterium probably loses its pathogenetic potential in the absence of tonsillar tissue [14].
Several manifestations of FB have been described. Before antibiotic era, a “subacute” variant comprising recurrent septicemic episodes was noted [1]. The “late metastatic” variant, consists of a single shower of bacteremia following a throat infection and the patients present with abscesses or septic arthritis weeks or months later. Lemierre described a short duration septicemia followed by a rapid recovery, known as “ephemeral bacteremia”, which he considered a relatively benign condition [15]. In consistence with the latter pattern our patient could have suffered transient/recurrent episodes of postanginal “occasional Fn bacteremia” that were selfrestricted with the microorganism being chronically nested into the tonsil crypts.
A case very similar to ours was reported by Batty et al [4]. They studied whether the same or different types of Fn were responsible for both CRT and systemic disease/LS. Their index case was a 41 years old female who presented with the acute phase of a CRT which had experienced since suffering mononucleosis at the age of 17, as our patient, occasionally manifested as high fever and tonsillar lesions. On this occasion, they isolated a heavy growth of Fn from a throat swab. The critical difference of Batty’s index case compared to ours is that, the former, didn’t develop metastatic disease, and no articular symptoms were reported in the history. They isolated Fn from 21% of CRT cases and concluded that similar strains are able to cause either chronic local or acute systemic disease suggesting that genetic factors such as those related to MHC class may influence the outcome of the disease in each patient.
PR is clinical syndrome characterized by recurrent flares of pain and swelling in and around joints (metacarpophalangeal, Proximal Interphalangeal (PIP), wrists, knees, and shoulders) that last for a few days and resolve spontaneously, as in our patient, and, unlike RA, does not produce chronic arthritis or radiographic images. Gout and Familial Mediterranean Fever (FMF) are also characterized by acute episodes of self-limiting joint and periarticular inflammation, thus, implying that PR flares might be related to inflammasome driven inflammation [16]. Canette et al., investigated whether PR patients carried mutation in the MEFV gene responsible for FMF. They identified that 8/65 of them carried at least one mutated of the four MEFV alleles. Authors suggested that in the presence of a triggering factor, these mutations may be responsible for the PR phenotype being similar but milder than that of FMF [17]. Several authors claim that PR represents variant of RA [16]. In a study that followed-up PR patients for the longest period ever (20 years), they found that 40/60 of them had developed RA [18]. The homozygosity for HLADR shared epitopes alleles was the only significant independent genetic risk factor for PR progression to chronic arthritis a similarity that suggests shared immunogenetic risk factors between the two conditions [18].
The question, if a CRT case can induce PR with auto-antibody production, is not known. Articular lesions are among the most common symptoms of LS/FB [1,6-9,19]. Lemierre [15], mentioned them as “extremely frequent” ranging from simple pain in the joints to suppurative arthritis. Reactive arthritis (ReA) on the other hand, has been defined as an acute sterile arthritis developing soon after or during an infection elsewhere in the body and with the live microorganism not entering the joint. Kobayashi et al, described ReA induced by CRT, with pyrexia and arthritis spanning from 2 weeks to 10 years [20], and were treated with antibiotics or tonsillectomy. Surprisingly, recurrence of arthritis was not recorded after tonsillectomy. More recently, Kobayashi et al., reported that one third of ReA cases demonstrated ANA and anti-cardiolipin positivity and another 10% RF positivity. ReA also ceased after tonsillitis treatment (antibiotics and/or tonsillectomy) and no recurrence was found [21]. Kawano et al., described a female with RA, and CRT initially well-controlled with treatment, who worsened. Closer examination revealed that the period of CRT exacerbation paralleled those of systemic arthritis [22]. Six days after tonsillectomy her serum RF levels had halved. They used TCR analysis to examine the infiltrating T cell clones in tonsils and synovium, and they found clonally common T cell responses in the two tissues, implying the existence of a common antigen, or molecular mimicry between synovial antigen and some kind of bacterial antigen that induces CRT [22].The bacterial antigen in our case could have originated from Fn.
Tonsillar Focal Infection (TFI), is discussed when CRT causes diseases in another distant organ, mostly including palmoplantar pustulosis (PPP), IgA nephropathy (IgAN), and RA. Meng et al, found in histologically distinctive tonsils with TFI, reduced reticulization of the tonsillar crypt epithelium, enlarged primary T nodules which play a major role in antigen triggering, helper T-cell-dependent stimulation, and subsequent maturation of antigen-responsive B cells into antibody-secreting plasma cells, compared to tonsils with simple CRT. These unusual immune responses in tonsils may underlie the pathogenesis of TFI. They also found elevated CD8+ T cells in the tonsils of patients with PPP, IgAN, and RA than in simple CRT patients that may migrate to distant organs and then drive lesions consistent with TFI [23].
Temoin et al., examined the presence of oral cavity bacterial DNA in synovial fluids of RA and OA patients, by using universal primers allowing the detection of a wide variety of microorganisms, excluding individuals being treated with antibiotics. They detected bacterial DNA in 5/36 patients’ joints with Fnuc being most commonly identified. Examining the source of Fnuc in the synovial fluid they found identical clones in patients’ plaque sample to the clonal level, thus providing unambiguous evidence of oral-joint spread. They postulated that the dramatic increase of oral bacteria in periodontitis patients along with an inflamed gum increases the chance of oral bacteria entering the circulation [24], in agreement with previous studies that Fnuc can translocate hematogenously from mother’s oral cavity to her pregnant uterus [25]. Other possible mechanisms implicated in the interrelationship between periodontitis and RA include i) inflammatory mediators locally released in the periodontium and distributed via the circulation, and bacteriallyinduced immunological dysregulations leading to autoimmunity, ii) swallowing and aspiration of the oral biofilm. Schmickler et al., evaluated the periodontal conditions and microbiological findings (PCR) and their influence on rheumatological disease parameters in patients with RA excluding individuals who had received antibiotics. Microbiological findings were similar within RA and control comprising 11 periodontopathogenic bacteria with Fnuc being most commonly identified. However, Fnuc and Peptostreptococcus micros were statistically significant in higher concentrations in RA, and there was a statistical significant association between RF and periodontitis severity. They concluded that, within their study, the assumed causal relations between periodontitis and RA were further substantiated [26].
It been recognized that RF response is transiently associated with many infections including; bacterial, as endocarditis (Bacteremia), Chlamydia pneumoniae, Klebsiella pneumoniae, Lyme, Syphilis; viral, as CMV, EBV, HAV, HBV, HCV, Dengue fever, HSV, HIV, Coxsackie B, Measles, Parvovirus, Rubella; parasitic, as Malaria, Toxoplasmosis, Chagas. Its presence can be associated with transient arthralgia and the net impact of the RF is to contribute to the host defense [27]. RF has been also detected in the gingiva, subgingival plaque, saliva and serum of periodontally diseased patients [28]. The RF periodontitis subjects show significantly elevated serum IgG and IgM antibody levels to oral microorganisms of the Capnocytophaga gingivalis species and Fnuc, which appear to express surface antigen epitopes that are antigenically related to determinants on IgG and can, induce cross-reactive IgM-RF [27]. They concluded that RF detected in the serum of some periodontitis patients may be elicited by certain microorganisms in the subgingival plaque.
Besides the RF production our patient had positive anti-SMA/ anti-f actin, and anti-CCP auto-antibodies. Anti-SMA and anti-F actin have been reported positive in long-lasting (months) cases of leishmaniasis [29], as well as in Q fever and in CMV infections [30,31], thus providing another connection between infectious agents and auto-antibody production. Furthermore, a systemic review ascertained positive anti-CCP in patients with tuberculosis (recording reduction in their titers after treatment!), HBV and HCV, leishmaniasis, Lyme, Chagas, Hansen disease, mononucleosis, Yersinia, schistosomiasis, HIV and HTLV-1 [32]. Remarkably, Makrygiannakis et al [33], studied the presence of citrullinated proteins in inflammatory conditions including RA, polymyositis and tonsil tissues. They concluded that all tonsil samples were positive with citrullinated proteins and PAD-2 (peptidylarginine deiminase) expression in macrophage-like cells, both in T cell and B cell areas, thus indicating a direct association between CRT and induction of autoimmunity with anti-CCP production, a finding that can be directly applied to our case.
After Lemierre’s original paper the widespread introduction of penicillin resulted in sparsely reported LS cases in the 1950s-1990s and authors referred to it as “the forgotten disease” [1,7,19]. During the last decades however, LS with all its variants, have been reported more frequently in Western countries [9]. This resurgence is attributed to the reduced antibiotic treatment owned to stewardship efforts and guidelines recommendations [1,3,9]. In addition, increased use of macrolide antibiotics for URT which are inactive against Fn, as well as fewer tonsillectomy procedures since 1970s, have also been incriminated [1,3,8,9]. In a much-publicized study, Centor et al [34] compared throat swabs from acute sore throat to asymptomatic controls, tested by molecular methods. Fn was detected significantly more often in symptomatic students (20.5% vs 9.4%), and more frequently than GAS (20.5%vs10.3%). Collectively, they reasoned their previous call for reconsideration of recommendations in current pharyngitis guidelines targeting, testing, and treatment of GAS only [36].
After conducting a meticulous literature review and in agreement with Centor, we could reason that Fn is probably the first cause of chronic as well as acute tonsillitis, when performed studies 1) exclude the use of antibiotics before admission, 2) swabs are drawn carefully by tonsillar crypts, and 3) molecular techniques are applied for its detection. Metronidazole is well-tolerated for decades, of low cost, and should be considered in CRT, taken into account the life-threatening complication of Necrobacillosis. Finally, the issue of tonsillectomy in CRT, as in our case would permanently eradicate Fn from tonsillar crypts and scars, thus should be reconsidered.
We present a middle-aged woman with CRT paralleled with PR and auto-antibody induction, complicated by Necrobacillosis. The combination of CRT with PR urged us to conduct a literature review focused on CRT pathogenesis and the subsequent autoimmunity induction. Many studies solely consider the disease parameters from the isolated interest of their own specialty, such as Internal Medicine, Infectious Diseases, Rheumatology, ENT, Dentistry, and Epidemiology. Collectively, we postulate that CRT and PR are aspects of the same disease entity. Moreover, we presented several studies straightly connecting CRT with typical RA, succeeding remission after tonsillectomy, with clonally common T cell responses in the two tissues, thus implying the existence of a common antigen, or molecular mimicry between synovial antigen and some kind of a bacterial antigen that induces CRT [22]. In agreement with that, oral cavity bacterial DNA, with Fnuc (80%) being the most common, was identified in patients’ joint suffering RA or Osteoarthritis [24]. Remarkably, Fnuc was identified in the 100% of periodontitis cases with RA, and was observed in higher concentrations in anti-CCP positive RA patients [26]. And the answer to the question, why gingivitis and periodontitis data should interest our case, is that tonsillar crypts, constitute a unique habitat, more reminiscent of the periodontal microbiota, and our patient underwent extended dental works a month before Necrobacillosis [12]. The laborious dental works could be the priming event that led the CRT to Necrobacillosis by altering the oral microbiota and thus affecting tonsils flora, as an alternative scenario to the preceding Mycoplasma infection mentioned above.
References
- Riordan T. Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre’s syndrome. Clin Microbiol Rev. 2007; 20: 622-659.
- Goldberg EA, Venkat-Ramani T, Hewit M, Bonilla HF. Epidemiology and clinical outcomes of patients with Fusobacterium bacteraemia. Epidemiol Infect. 2013; 141: 325-329.
- Osowicki J, Kapur S, Phuong LK, Dobson S. The long shadow of Lemierre’s syndrome. J Infect. 2017; 741: S47-S53.
- Batty A, Wren MW, Gal M. Fusobacterium necrophorum as the cause of recurrent sore throat: comparison of isolates from persistent sore throat syndrome and Lemierre’s disease. J Infect. 2005; 51: 299-306.
- Ludlam H, Howard J, Kingston B, Donachie L, Foulkes J, Guha S, et al. Epidemiology of pharyngeal carriage of Fusobacterium necrophorum. J Med Microbiol. 2009; 58: 1264-1265.
- Kristensen LH, Prag J. Lemierre’s syndrome and other disseminated Fusobacterium necrophorum infections in Denmark: a prospective epidemiological and clinical survey. Eur J Clin Microbiol Infect Dis. 2008; 27: 779-789.
- Nohrström E, Mattila T, Pettilä V, Kuusela P, Carlson P, Kentala E, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011; 43: 463-470.
- Kuppalli K, Livorsi D, Talati NJ, Osborn M. Lemierre’s syndrome due to Fusobacterium necrophorum. Lancet Infect Dis. 2012; 12: 808-815.
- Kristensen LH, Prag J. Human Necrobacillosis, with emphasis on Lemierre’s syndrome. Clin Infect Dis. 2000; 31: 524-532.
- Li Y, Yue J, Yang C. Unraveling the role of Mg (++) in osteoarthritis. Life Sci. 2015; 147: 24-29.
- Jensen A, Kristensen LH, Prag J. Detection of Fusobacterium necrophorum subsp. funduliforme in tonsillitis in young adults by real-time PCR. Clin Microbiol Infect. 2007; 13: 695-701.
- Jensen A, Fagö-Olsen H, Sørensen CH, Kilian M. Molecular mapping to species level of the tonsillar crypt microbiota associated with health and recurrent tonsillitis. PLoS One. 2013; 8: e56418.
- Dapefrid A, Lundström B, Tano K. Prevalence of Fusobacterium necrophorum in tonsils from patients with chronic tonsillitis. Acta Otolaryngol. 2017; 137: 297-301.
- Björk H, Bieber L, Hedin K, Sundqvist M. Tonsillar colonisation of Fusobacterium necrophorum in patients subjected to tonsillectomy. BMC Infect Dis. 2015; 15: 264.
- Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936; 2: 701-703.
- Mankia K, Emery P. What can palindromic rheumatism tell us? Best Pract Res Clin Rheumatol. 2017; 31: 90-98.
- Cañete JD, Arostegui JI, Queiró R, Gratacós J, Hernández MV, Larrosa M, et al. An unexpectedly high frequency of MEFV mutations in patients with anti-citrullinated protein antibody-negative palindromic rheumatism. Arthritis Rheum. 2007; 56: 2784-2788.
- Maksymowych WP, Suarez-Almazor ME, Buenviaje H, Cooper BL, Degeus C, Thompson M, et al. HLA and cytokine gene polymorphisms in relation to occurrence of palindromic rheumatism and its progression to rheumatoid arthritis. J Rheumatol. 2002; 29: 2319-2326.
- Brazier JS, Hall V, Yusuf E, Duerden BI. Fusobacterium necrophorum infections in England and Wales 1990-2000. J Med Microbiol. 2002; 51: 269- 272.
- Kobayashi S, Tamura N, Akimoto T, Ichikawa G, Xi G, Takasaki Y, et al. Reactive arthritis induced by tonsillitis. Acta Otolaryngol Suppl. 1996; 523: 206-211.
- Kobayashi S, Ichikawa G. Reactive arthritis induced by tonsillitis: a type of ‘focal infection’. Adv Otorhinolaryngol. 2011; 72: 79-82.
- Kawano M, Okada K, Muramoto H, Omura T, Inoue R, Kitajima S, et al. Simultaneous, clonally identical T cell expansion in tonsil and synovium in a patient with rheumatoid arthritis and chronic tonsillitis. Arthritis Rheum. 2003; 48: 2483-2488.
- Meng HX, Ohe R, Li HN, Yang SR, Kabasawa T, Kato T, et al. Immunoglobulin and CD8 T-cell distribution in histologically distinctive tonsils of individuals with tonsillar focal infection. Acta Otolaryngol. 2015; 135: 264-270.
- Témoin S, Chakaki A, Askari A, El-Halaby A, Fitzgerald S, Marcus RE, et al. Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol. 2012; 18: 117-121.
- Han YW. Can oral bacteria cause pregnancy complications? Womens Health (Lond). 2011; 7: 401-404.
- Schmickler J, Rupprecht A, Patschan S, Patschan D, Mμller GA, Haak R, et al. Cross-Sectional Evaluation of Periodontal Status and Microbiologic and Rheumatoid Parameters in a Large Cohort of Patients With Rheumatoid Arthritis. J Periodontol. 2017; 88: 368-379.
- Newkirk MM. Rheumatoid factors: host resistance or autoimmunity? Clin Immunol. 2002; 104: 1-13.
- Thé J, Ebersole JL. Rheumatoid factor from periodontitis patients’ crossreacts with epitopes on oral bacteria. Oral Dis. 1996; 2: 253-262.
- Makaritsis KP, Gatselis NK, Ioannou M, Petinaki E, Dalekos GN. Polyclonal hypergammaglobulinemia and high smooth-muscle autoantibody titers with specificity against filamentous actin: consider visceral leishmaniasis, not just autoimmune hepatitis. Int J Infect Dis. 2009; 13: e157-160.
- Cunha BA, Nausheen S, Busch L. Severe Q fever Community-Acquired Pneumonia (CAP) mimicking Legionnaires’ disease: Clinical significance of cold agglutinins, anti-smooth muscle antibodies and thrombocytosis. Heart Lung. 2009; 38: 354-362.
- Bonnet F, Morlat P, Neau D, Viallard JF, Ragnaud JM, Dupon M, et al. [Hematologic and immunologic manifestations of primary cytomegalovirus infections in non-immunocompromised hospitalized adults]. Rev Med Interne. 2000; 21: 586-594.
- Lima I, Santiago M. Antibodies against cyclic citrullinated peptides in infectious diseases--a systematic review. Clin Rheumatol. 2010; 29: 1345-1351.
- Makrygiannakis D, af Klint E, Lundberg IE, Löfberg R, Ulfgren AK, Klareskog L, et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006; 65: 1219-1222.
- Centor RM, Atkinson TP, Ratliff AE, Xiao L, Crabb DM, Estrada CA, et al. The clinical presentation of Fusobacterium-positive and streptococcal-positive pharyngitis in a university health clinic: a cross-sectional study. Ann Intern Med. 2015; 162: 241-247.
