Abstract
Background: GD is a multifactorial disease that is triggered by multiple genes and multiple factors. Genetic variation is correlated to 79% GD susceptibility and some reports explained that genetic factors also affected the occurrence of recurrence. This study aimed to evaluate the role of genetic factors affecting the relapse of patients with Graves’ disease in Indonesia, included polymorphism of exon 1 CTLA4 (49) A/G rs231775, promoter CTLA4 (-318) C/T, intron 1 CTLA4 (1822) C/T, CT60 (+6230) G/A rs3087243, exon 33 thyroglobulin C/T, intron 1 TSHR C/T and 5’-UTR CD40 C/T.
Methods: This was a retrospective study that were collected from 256 GD patients, 128 GD patients who recurred and 128 GD patients who did not relapse after no longer taking anti thyroid drugs for more than 1 years. Genetic polymorphism examination was performed using PCR-RFLP. The logistic regression was used since the dependent variables were categorical variables.
Results: the analysis of this study demonstrated that there was a correlation between relapse of disease and age at diagnosis (p=0.003), family factors (p=0.001), smoking (p=0.005), G/G genotype of CTLA-4 gene on the nucleotide 49 at codon 17 of exon 1 (p=0.002), C/C genotype of promoter -318 CLTA4 gene (p=0.003), C/C genotype of TSHR gene on the rs2268458 of intron 1 (p=0.003) and C/C genotype of thyroglobulin gene on exon 1 (p=0.012).
Conclusion: This study confirms the usefulness of the exon 1 CLTA4 G/G genotype, promoter -318 CLTA4 C/C genotype and intron 1 TSHR C/C genotype in predicting recurrence after cessation of treatment, and may not be good candidates for antithyroid drugs.
Keywords: Graves’ Disease; Recurrence; CTLA-4 Gene; TSHR Gene; Thyroglobulin gene; CD40 gene
Introduction
Hyperthyroidism is one of the forms of thyrotoxicosis caused by increased synthesis and secretion of thyroid hormones by the thyroid gland. The term hypertiroid disease needs to be distinguished from thyrotoxicosis. Graves' Disease (GD), also known as toxic diffuse goiter, can increase the level of thyroid hormone, is one of the organ specific autoimmune diseases and it accounts for 85% of all clinical hyperthyroidism. GD is inherited as a complex multigenic disorder, with the presence of antibodies acting as agonists against the thyrotropin receptor in thyroid gland [1]. GD often presents in patients aged from 20-40 years old, with a male to female ratio of ~1:8 and a significant familial tendency. In America, prevalence of GD is 1%, Caucasians population about 0.5% to 2% [2,3]. In Indonesia, based on Indonesia Basic Health Survey (Riskesdas) 2013 showed 0.4% of Indonesian population aged over 15 years was diagnosed with hyperthyroidism. Although in a small percentage, but the quantity is quite large that about more than 500,000 people of Indonesia with hyperthyroidism [4]. Immunologically, GD is characterized by increased circulating antibodies against Thyroid-Stimulating Hormone Receptor (TSHR), Thyroglobulin (TG) and Thyroid Peroxidase (TPO). Although the precise pathogenesis involved in the process of GD is not completely understood, certain findings indicate that complex interactions between environmental, genetic, endogenous and local factors are involved in its pathogenesis. Genetic variation is correlated to 79% GD susceptibility and some reports explained the relationship between gene polymorphisms and the recurrence risk in GD patients after ATD withdrawal [4,5].
The treatment option of GD is antithyroid drugs, radioactive iodine or surgery. Antithyroid Drugs (ATDs) are considered as the first choice therapy for GD patients. However, GD patients treated with ATD often relapses after discontinuation of anti-thyroid drugs; only about 50% of patients will achieve complete remission. The persistent or recurrent hyperthyroidism results in increased medical expenses and a wide spectrum of complications, such as atrial fibrillation, heart failure, and osteoporosis, even a long-term and negative impact on the quality of life. Thus, it is of great importance to identify some predictors, prior to ATD treatment, which can indicate a higher risk of recurrence. If the likelihood of recurrence after ATD treatment is high, radioactive iodine therapy or thyroidectomy might be more preferable. The evidence from further genetic studies showed that genetic factors are not only related to the development of GD but also the predictors for the recurrence risk in GD patients after ATD withdrawal [6].
Recently, we investigate the predictive value of gene polymorphisms with higher recurrence risk in GD. The follow-up duration was 24-36 months after withdrawing therapy. Indeed, several SNPs of gene within the exon 1 CTLA4 (+49) A/G rs231775, promoter CTLA4 (-318) C/T rs5742909, CT60 (+6230) G/A rs3087243, intron 1 TSHR C/T, exon 33 thyroglobulin C/T and 5’-UTR CD40 C/T had been analyzed in this study. This strategy would facilitate an appropriate therapeutic approach for a given patient at the time of GD diagnosis.
Methods
This was a retrospective analysis of data that were collected from 256 GD patients, 205 females and 51 males; aged 45 ± 17 year, recruited from the Endocrine Clinic of Cipto MangunKusumo Hospital Jakarta. The subjects in this study were 128 GD patients who recurred and were referred to as the case group, and 128 GD patients who did not relapse after no longer taking antithyroid drugs for more than 1 year and were declared as the control group. The selection of case group and control group based on inclusion and exclusion criteria.
The diagnosis criteria for GD were elevated serum T4 and/or T3 and suppressed TSH levels; and or diffusely increased thyroidal uptake of technetium-99 m; and or the presence of TSH- receptor antibodies. Only patients who completed a treatment course of 1 to 3 years and had adequate follow-up after drug withdrawal were included. Patients with a history of radioiodine therapy or previous thyroid surgery were excluded. Relapse was confirmed by the recurrence of symptoms of hyperthyroidism and laboratory data of elevated serum T4 and/or T3 exceeding the upper limit of the normal range, and suppressed serum TSH. This study has been granted the permission of Research Ethics Committee of Medical Faculty of Indonesia No.490/UN2.F1/ETIK/2015 and 204 /UN2.F1/ETIK/2018. All data and research results were kept confidential.
DNA was extracted from peripheral blood leukocytes and isolated using the salting out method with reagents derived from the Promega Wizard DNA Purification Kit (Ref A1125). The procedure of DNA isolation was performed in accordance with the measurement of DNA concentration and purity used spectrophotometer (nanodrop, Maestro) with a purity range of 1.8 to 2.0 of the kit. DNA amplification using the EpiTect MSP kit reagent. The PCR-HRM curve was used to genotype SNPs, adopting a 10-μl PCR reaction assay system containing 5 μl Premix Taq™, 0.2 μM of each primer, 50 ng DNA, and sterile water. The following reaction conditions were applied: 3 min denaturation at 94°C, followed by 30 cycles of a denaturation step (30 seconds at 94°C), an annealing step (30 seconds at annealing temperature), and an elongation step (30 seconds at 72°C), and a final elongation reaction at 72°C for 10 min. Table 1 lists the PCR primers and enzyme restriction endonuclease of each SNP. Amplification performed in 35 cycles and after the amplification process, 5 mL of 25 mL PCR reaction was restricted for 3 hours at 37°C. Result of amplification separated by electrophoresis on 2% agarose with 90 volts for 60 minutes. Visualization of the band of DNA fragments of electrophoresis results was observed with a UV illuminator (UV long lifeTM filter Spectroline) (Figure 1) [7].
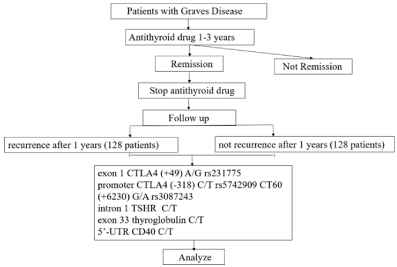
Figure 1: Flow chart of study.
Statistical Analysis
Comparisons of individual clinical and laboratory variables between group’s case and control were performed with χ2 test or Fisher exact test for the categorical data. Multivariate logistic regression was performed to assess the strength of association between the length of remission and the clinical and laboratory variables. The Odds Ratio (OR) and its 95% Confidence Intervals (CI) were calculated by using SPSS version 22 for Windows. A two-tailed P value less than 0.05 was considered statistically significant.
Results
There were 128 patients in case group and 128 patients in control group. The distribution of allele and genotype frequencies of CTLA4 gene, thyroglobulin gene, TSHR gene and CD40 gene in patients GD patients with relapse and GD patients without relapse is shown in Table 2. There was a significant difference of genotype distribution (P=0.001) among the two groups (Figure 2a,2b,2c,2d,2e and Figure 2f).
SNP
Primer
Size (bp)
Enzyme restriction endonuclease
Exon 1 CTLA4 (49) rs231775
FGGCTTGCCTTGGATTTCAACGGC RGCTTCCAAAAAGTCTCACTCACC
129 (98/41)
Fnu4HI
Promoter CTLA4 (-318) rs5742909
FAAATGAATTGGACTGGATGGT RTTACGAGAAAGGAAGCCGTG
247 (132/115)
MseI
3'-UTR CT60 (+6230) rs3087243
FATAATGCTTCATGAGTCAGCTT RGAG GTGAAGAACCTGTGTTAAA
178 (107/71)
MaeII
Intron 1 TSHR
FCCAGCAGAGGGAGCACAA RTAGAGAATAGAGCAGCAAGACACT
608 (275/333)
AluI
Exon 33 thyroglobulin (5995)
FATATTGACCAAAGCACCCCC RATTAGCCAGTTGCCCTCTCC
375 (167/208)
Hpy99I
5'-UTR CD40
FTGGGAATGTTCTGGGGAA RAGGCCTCTTCCCCGAA
320 (184/136)
NcoI
Table 1: SNP PCR primers.
Variable
Recurrence (=128)
Non recurrence (=128)
Total (=256)
P
OR (Confidence Interval)
Sex (n, %)
Female
110 (42.9)
95 (37.1)
205 (80.1)
0.41
0.71 (0.28-1.83)
Male
18 (7.1)
33 (12.9)
51 (19.9)
Age at diagnosis (years)
Mean
28.6
38.3
33.4
0.01
< 30 years
89 (34.8)
34 (13.4)
123 (48.1)
0
1.78 (0.91-4.19)
? 30 years
39 (15.2)
94 (36.6)
133 (51.9)
Family History
With family history
74 (28.9)
25 (9.8)
99 (38.7)
0
2.14 (1.21-3.78)
No family history
54 (21.1)
103 (40.2)
157 (61.3)
Smoking or history of smoking
Smoking
20 (7.8)
2 (0.8)
22 (8.6)
0.01
2.37 (0.18-3.34)
No smoking
108 (42.2)
126 (49.2)
234 (91.4)
exon 1 CTLA4 (+49) A/G rs231775
Genotype GG
68 (26.5)
21 (8.2)
89 (34.8)
0
5.31 (1.43-17.2)
Genotype GA
39 (15.2)
57 (22.2)
96 (37.5)
0.06
1.43 (0.32-6.49)
Genotype AA
21 (8.3)
50 (19.6)
71 (27.7)
Allele G
175 (34.2)
99 (19.3)
274 (53.5)
0
4.84 (1.18-10.9)
Allele A
81 (15.8)
157 (30.7)
238 (46.5)
promoter CTLA4 (-318) C/T rs5742909
Genotype CC
88 (34.4)
64 (25)
152 (65.1)
0
3.25 (2.13-6.34)
Genotype CT
31 (12.1)
33 (12.8)
64 (25)
0.01
2.12 (2.11-5.45)
Genotype TT
9 (3.5)
31 (12.2)
40 (9.9)
Allele C
207 (40.4)
161 (31.4)
368 (71.9)
0
6.31 (3.41-12.8)
Allele T
49 (9.6)
95 (18.6)
144 (28.1)
3'-UTR CT60 (+6230) rs3087243
Genotype GG
107 (41.9)
74 (28.9)
181 (70.7)
0.11
1.8 (0.78-4.41)
Genotype GA
18 (6.9)
52 (20.3)
70 (27.4)
Genotype AA
3 (1.2)
2 (0.8)
5 (1.9)
Allele G
232 (45.3)
200 (39.1)
432 (84.4)
0.21
1.7 (0.74-3.92)
Allele A
24 (4.7)
56 (10.9)
80 (15.6)
intron 1 TSHR C/T rs2268458
Genotype CC
56 (21.9)
39 (15.2)
95 (37.1)
0
3.3 (0.45-9.41)
Genotype CT
54 (21.1)
64 (25)
118 (46.1)
0.06
2.1 (0.73-7.12)
Genotype TT
18 (7.0)
25 (9.8)
43 (16.8)
Alel C
166 (32.4)
142 (27.8)
308 (60.2)
0
5.2 (1.47-13.41)
Alel T
90 (17.6)
114 (22.2)
204 (39.8)
exon 33 thyroglobulin C/T
Genotype CC
38 (14.8)
10 (3.9)
48 (18.8)
0.01
4.1 (1.78-13.6)
Genotype CT
85 (33.3)
82 (32.0)
167 (65.2)
0.03
2.5 (0.82-8.13)
Genotype TT
5 (1.9)
36 (14.1)
41 (16.0)
Allele C
161 (31.4)
102 (19.9)
263 (51.4)
0.03
2.7 (1.09-7.56)
Allele T
95 (18.6)
154 (30.1)
249 (48.6)
5'-UTR CD40 C/T
Genotype CC
14 (5.5)
2 (0.8)
16 (6.3)
0.11
1.45 (0.98-2.32)
Genotype CT
110 (42.9)
108 (42.2)
228 (89.1)
0.32
0.97 (0.64-1.56)
Genotype TT
4 (1.6)
18 (7.0)
22 (8.6)
Allele C
138 (26.9)
112 (21.9)
250 (48.8)
0.37
1.31 (0.32-3.45)
Allele T
118 (23.1)
144 (28.1)
262 (51.2)
Table 2: Characteristic of Subjects.
Variable
Adjusted OR
P value
95% CI
Age at diagnosis < 30 year
2.104
0.031
0.930-5.085
Family history of thyroid disease
1.239
0.042
0.971-6.129
Smoking
2.222
0.036
1.138-5.368
Genotype G/G CTLA4 (+49) rs231775
3.963
0.022
1.755-7.963
Genotype C/C CTLA4 (-318) rs5742909
2.264
0.037
0.335-5.762
Genotype C/C intron 1 TSHR rs2268458
2.682
0.031
0.114-7.078
Genotype C/C exon 33 thyroglobulin
1.095
0.095
0.631-4.215
Table 3: Multivariate logistic regression analysis for determinants of recurrence.
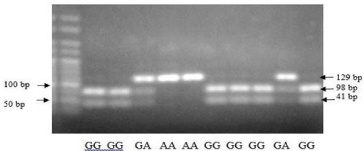
Figure 2a: PCR RFLP CTLA4 gene on nucleotide 49 at codon 17 of exon 1.
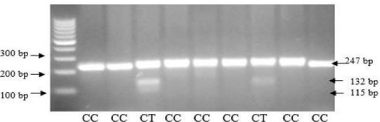
Figure 2b: PCR RFLP CTLA-4 gene of promoter -318 C/T rs5742909.
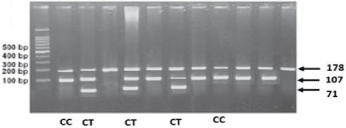
Figure 2c: PCR RFLP TSHR gene polymorphism CT60 (+6230) G/A
rs3087243.
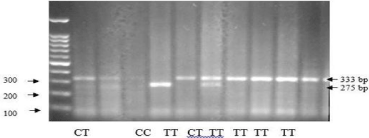
Figure 2d: PCR RFLP TSHR gene polymorphism rs2268458 of intron 1.
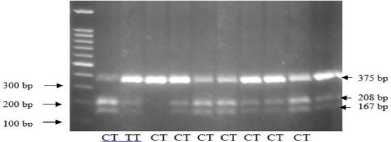
Figure 2e: PCR RFLP Thyroglobulin gene polymorphism of exon 33.
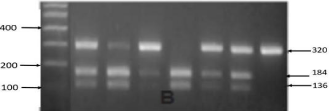
Figure 2f: PCR RFLP CD40 gene polymorphism of 5'-UTR.
Discussion
T-cell abnormality is associated with the pathogenesis of GD and cytotoxic T lymphocyte- associated protein-4 (CTLA-4) is an important negative-regulatory factor of T-cell mediated immune responses. The CTLA4 gene (CD152), as immune-regulatory molecule, is able to inhibit T-cell response while triggered by costimulatory molecules. CTLA4 inhibited T-cell activation and mediated antigen-specific apoptosis of T cells. CTLA4 expresses on the activated CD4+ and CD8+ T cells. It has sequence homology with costimulatory molecules, including CD28, but has an opposing role. CTLA4 produces inhibitory signals combining with the relevant molecules in the B7 family, which has an important role in the termination of T-cell activation. The abnormal expression of CTLA4 induces the activation of T cells, breaks down the immune tolerance and results in autoimmune diseases. This gene is located on human chromosome 2q33 and is composed of four exons. Full-length human CTLA4 gene spans 6.1 kb of genomic DNA and encodes 233 amino acids, with 4 exons and 3 introns [8]. Indeed, several SNPs within the CTLA4 gene (C/T polymorphism in the promoter region -318, A/G polymorphism in exon 1+49 (rs231775) and CT60 (rs3087243) in the 6.1-kb 3′ noncoding region have been suggested to be associated with the development of GD by several Genome-wide Association Studies (GWAS) (Figure 3).
Exon 1 CTLA4 (+49) gene A/G (rs231775) The exonic SNP rs231775 in the CTLA4 leader peptide results in threonine to alanine conversion in the signal peptide part which was reported to cause misprocessing of CTLA4, resulting in less efficient glycosylation and diminished surface expression of CTLA-4 protein.
Polymorphism decreased CTLA4 cell surface expression and reduced the inhibitory function of CTLA4, and the G allele reduced the ability to control T-cell proliferation. Therefore, this might confer a lesser CTLA4 function, resulting in greater T-cell activity, stronger immune response, and a higher probability of autoimmunity. There was a significant difference of genotype distribution of exon 1 CTLA4 (+49) (χ2 test, P = 0.002) among the two group. Recurrence group had the highest proportion (26.5%) of the G/G genotype compared with non-recurrence group (8.2%). Similarly, there was a clear trend of increasing frequency of the G allele (34.2%) in recurrence group and increasing frequency of the A allele (30.7%) in non-recurrence group (P = 0.001). Individuals with the exon 1 CTLA4 (+49) A allele have higher CTLA4 expression (Table 2) [10].
Promoter CTLA4 (-318) gene C/T (rs5742909)
A promoter is a DNA sequence where transcription of the DNA begins. The promoter controls the expression of the gene by signaling to RNA polymerase to begin transcription and the direction in which the polymerase should transcribe the DNA. The specific sequence of the promoter determines the strength of the promoter, means that a strong promoter leads to a high rate of transcription initiation. SNP -318 (rs5742909) is at position of the promoter region of the CTLA4 gene. For SNP promoter -318, there was a significant difference of genotype distribution (P=0.003) among recurrence and non-recurrence group. Recurrence group had the highest proportion (34.4%) of the C/C genotype compared with non-recurrence group (25%), and there was a trend of increasing frequency of the C allele in recurrence group (40.4%) and increasing frequency of the T allele in non-recurrence group (18.6%) [11].
CT60 (3'-UTR) CTLA4 gene C/T (rs3087243)
SNP 3’-UTR CT60 (rs3087243) in the CTLA4 gene is a complex region containing multiple binding sites for microRNA and located downstream of the poly (A) termination site. Presence of SNP or base mutation in the 3'-UTR would change the signaling pathway, influence the protein expression and result in diseases. Previous studies have suggested that this polymorphism is important for efficient splicing and production of soluble CTLA4 (sCTLA4), and play a role in the mRNA stability of sCTLA4. The soluble form of CTLA4 is translated and secreted in human serum and can bind to CD80/86 molecules. Recombinant sCTLA4 inhibits T-cell proliferation in vitro, indicating that a reduction in the levels of this form of CTLA4 could lead to inefficient blocking of the immune response, triggering an autoimmune disorder. In line with this notion, subjects with the putative risk G/G genotype have lower production of sCTLA4 than that by subjects with the protective A/A genotype [12]. It demonstrated hypothesis that CT60 of CTLA4 appears to be susceptible in GD. In this study there was no significant difference of genotype distribution of 3’-UTR CT60 among the two groups. Recurrence group had the highest proportion (41.9%) of the G/G genotype compared with non-recurrence group (28.9%), but there was a trend of increasing frequency of the G allele (45.3%) in recurrence group and increasing frequency of the A allele (10.9%) in non-recurrence group (P=0.214). However there was no significant difference identified between GD patients with relapse history, the CT60 gene polymorphism of CTLA4 may not be associated with the progress of GD.
Intron 1 TSHR gene C/T (rs2268458)
TSHR is a primary candidate gene believed to be related to GD susceptibility. The human TSHR gene occupies 191 kb of DNA and is located at chromosome 14q31. TSHR is a specific protein expressed in thyroid cells in the thyroid follicular membrane. TSH regulates both thyroid growth and functionality via TSHR signaling. TSHR is a member of the G-protein-coupled receptor super family encoded on chromosome [13,14]. The protein is a single 764 amino acid peptide chain encoded for across 10 exons with a molecular weight of 84000 Daltons. TSH binding to TSHR promotes G-protein signaling, leading to activation of the cAMP and/or phosphoinositide Ca2+ signal transduction pathways [13].
Introns are the noncoding DNA sections, located between coding regions of the gene termed exons, and they are spliced out from mRNA before translation. Exons only compose about 1.5% to 2% of the human genome, and intronic and intergenic DNAs make up the remaining human genome space. The total length of introns comprises about 37% of the human genome. Despite this, introns were generally thought of as junk DNA regions with no function after their recognition. However, recent evidence has indicated that introns contain important gene regulatory sequences that have a variety of functional roles, such as alternative intronic promoters/enhancers, noncoding RNAs, RNA editing, nested genes, and transacting elements. Sometimes, intronic mutations may even lead to diseases due to the modification of the mRNA splicing process. It is also well known that intron 1 of many genes contains certain regulatory cis-elements (transcription factor binding sites) [14]. The TSHR intron 1 is about 106 kb and occupies roughly 56% of the TSHR gene sequence. Intronic small RNAs may be involved in the regulation of mRNA splicing, and gene expression including the control of the immune response and may also potentially arise from intron 1 [15]. There was a significant difference of genotype distribution of TSHR intron 1 rs2268458 among the two groups. Recurrence group had the highest proportion (21.9%) of the C/C genotype compared with non-recurrence group (15.2%). Similarly, there was a clear trend of increasing frequency of the C allele (32.4%) in recurrence group and increasing frequency of the T allele (22.2%) in non-recurrence group (P 0.01).
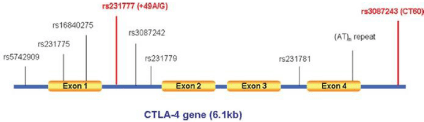
Figure 3: The human CTLA4 gene structure and known polymorphism [9].
Exon 33 Thyroglobulin
Human Thyroglobulin (TG) molecule has specific features, and one of the main autoantigens in GD. Thyroglobulin gene polymorphism in exon 33 (Tg E33SNP) was related to the increased susceptibility for GD and the recurrence risk of GD. The E33SNP C/C genotype of the TG gene associated with a subgroup of GD patients who were likely to have a higher relapse rate. Unlike the other putative AITD susceptibility genes e.g. CTLA-4 and CD40, which are immune regulators, TG is a thyroid-specific gene, which may explain the targeting of the autoimmune response to the thyroid gland and make it an excellent candidate for GD. Because CTLA-4 is a nonspecific costimulatory molecule, it is expected to confer susceptibility to AITD and autoimmunity in general, but not specifically to GD. The interaction of immunoregulatory genes and an autoantigen-specific gene may be a general mechanism related to the development of organ-specific autoimmune diseases [16].
In this study there was a significant difference of genotype distribution of exon 3 thyroglobulin gene among the two groups. Recurrence group had the highest proportion (14.8%) of the C/C genotype compared with non-recurrence group (3.9%). Similarly, there was a clear trend of increasing frequency of the C allele (31.4%) in recurrence group and increasing frequency of the T allele (30.1%) in non-recurrence group (P = 0.031).
5'-UTR CD40 gene C/T
CD40 is a tumor necrosis factor receptor (TNFR) that mainly expressed on antigen- presenting cells and B cells, as well as on other types of cells, such as thyroid follicular cells (thyrocytes). CD40 not only interacts with the CD40 ligand (CD40L) on the T cells, but also promotes B-cell proliferation and antibody secretion. CD40 has emerged as a key genetic player in the development of GD and autoimmunity in general. CD40 is required for an effective adaptive immune response. The interaction with its ligand, CD154 (expressed on T cells), provides the needed costimulatory signals to stimulate B cell proliferation, immunoglobulin class switching, and germinal center formation. CD40’s central role in coordinating T cell and B cell mediated immunity is underscored by the profound immune system aberrations that develop with CD40 over-activity in GD. This association was signi?cantly stronger in a subset of Graves’ patients having high titers of thyroid speci?c antibody, suggesting that CD40 played a role in the production of antibody that mediates GD [17]. Thyroidal CD40 Overexpression augments the production of thyroid-speci?c antibody, resulting in a more severe disease, whereas deletion of thyroidal CD40 had the opposite effect. One of the major effects of CD40 stimulation is induction of cytokine secretion. Thyroidal CD40 stimulation results in local cytokine secretion as IL-6, TNF-a, and IL- 8 (Figure 4) [18,19].
In this study there was no significant difference of genotype distribution of 5’-UTR CD40 gene among the two group (P =0.113), but recurrence group had the highest proportion (5.5%) of the C/C genotype compared with non-recurrence group (0.8%), and also the trend of increasing frequency of the C allele (26.9%) in recurrence group and increasing frequency of the T allele (28.1%) in non-recurrence group.
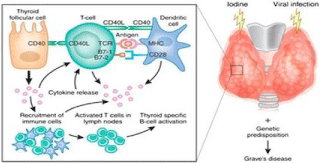
Figure 4: Proposed mechanism of the function of CD40 in the pathogenesis
of GD [19].
Multivariate analysis
In this study we used remission for more than 1 year to dichotomize remission duration after cessation of treatment, while performing multivariate logistic regression analysis.
The results showed that patient with age at diagnosis less than 30 years old, had family history of thyroid disease and did not quit smoking during the treatment, had a risk for relapse. The GG genotype of exon1 49 CLTA4, CC genotype of promoter CTLA4 and CC genotype of intron 1 TSHR are significant predictors to recurrence (Table 3).
Conclusion
This retrospective case control study in Indonesia population of the 256 GD patients with 128 recurrences and 128 non recurrences showed that CTLA-4, TSHR and thyroglobulin gene were correlated with recurrence after discontinuation of antithyroid drugs. GD is an autoimmune disease and is thought to be caused by several genetic factors, that no single gene is necessary for its development. The GD patients with exon 1 CLTA4 G/G genotype, promoter -318 CLTA4 C/C genotype and intron 1 TSHR C/C genotype, may not be good candidates for antithyroid drugs.
The predictive value of gene polymorphisms for the high recurrence risk in GD patients is still some query. Further prospective studies with large sample sizes and long-term follow-up, should be conducted to confirm the predictive roles of gene polymorphism. Despite these conflicting results, one implication is that gene polymorphisms are supposed to an important risk predictor for the recurrence risk in GD patients. In addition, it is worth noting that the etiology of GD is related to genetic and environmental factors, so it is difficult for any single factor to well predict the recurrence risk for a given patients. Thus, a prediction model based on both genetic and environmental risk factors for GD recurrence might better predict the recurrence risk for a given patient.
References
- Dong YH, Fu DG. Autoimmune thyroid disease: mechanism, genetics and current knowledge. Eur Rev Med Pharmacol Sci. 2014; 18: 3611-3618.
- McLeod DSA, Cooper DS, Ladenson PW, Whiteman DC, Jordan SJ. Race/ethnicity and the prevalence of thyrotoxicosis in young Americans. Thyroid. 2015; 25: 621-628.
- Indonesia Basic Health Survey. Ministry of Health Republic of Indonesia. 2013.
- Hemminki K, Li X, Sundquist J, Sundquist K. The epidemiology of Graves’ disease: evidence of a genetic and an environmental contribution. J Autoimmun. 2010; 34: J307-313.
- Lombardi A, Menconi F, Greenberg D. Dissecting the genetic susceptibility to Graves’ disease in a cohort of patients of Italian origin. Front Endocrinol (Lausanne). 2016; 7: 21.
- Shukla SK, Singh G, Ahmad S, Pant P. Infections, genetic and environmental factors in pathogenesis of autoimmune thyroid diseases. Microb Pathog. 2018; 116: 279-288.
- Gu LQ, Zhu W, Zhao SX, Zhao L, Zhang MJ, Cui B, et al. Clinical associations of the genetic variants of CTLA-4, Tg, TSHR, PTPN22, PTPN12 and FCRL3 in patients with Graves’ disease. Clin Endocrinol (Oxf). 2010; 72: 248-255.
- Alvarez-VAzquez P, Garcia-Mayor RV, Larranaga A, Valverde D. Association of CTLA-4 Gene Polymorphism with Ophthalmopathy of Graves’ Disease in a Spanish Population. Int J Endocrinol Metab. 2011; 9: 397-402.
- Tu Y, Fan G, Dai Y, Zeng T, Xiao F, Chen L, Kong W. Association between rs3087243 and rs231775 polymorphism within the cytotoxic T-lymphocyte antigen 4 gene and Graves’ disease: a case/control study combined with meta-analyses. Oncotarget. 2017; 8: 110614-110624.
- Ting WH, Chien MN, Lo FS. Association of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) gene polymorphisms with autoimmune thyroid disease in children and adults: case-control study. PLoS One. 2016; 11: e0154394.
- Shehjar F, Afroze D, Misgar RA, Malik SA, Laway BA. Association of FoxP3 promoter polymorphisms with the risk of Graves' disease in ethnic Kashmiri population. Gene. 2018; 672: 88-92.
- Fang W, Zhang Z, Zhang J, Cai Z, Zenh H, Chen M, et al. Association of the CTLA4 gene CT60/rs3087243 single-nucleotide polymorphisms with Graves' disease. Biomed Rep. 2015; 3: 691-696.
- Diana T, Daiber A, Oelze M, Neumann S, Olivo PD, Kanitz M, et al. Stimulatory TSH receptor antibodies and oxidative stress in Graves disease. J Clin Endocrinol Metabol. 2018; 103: 3668-3677.
- Bufalo NE, dos Santos RB, Marcello MA, Piai RP, Romaldini J, Ward L, et al. TSHR intronic polymorphisms (rs179247 and rs12885526) and their role in the susceptibility of the Brazilian population to Graves’ disease and Graves’ ophthalmopathy. J Endocrinol Invest. 2015; 38: 555-561.
- Qian W, Xu K, Jia W, Lan L, Zheng X, Yang X, et al. Association between TSHR gene polymorphism and the risk of Graves' disease: a meta-analysis. J Biomed Res. 2016; 30: 466-475.
- Hsiao JY, Hsieh MC, Tien KJ, Hsu SC, Shin SJ, Lin SR. Association between a C/T Polymorphism in Exon 33 of the Thyroglobulin Gene Is Associated with Relapse of Graves’ Hyperthyroidism after Antithyroid Withdrawal in Taiwanese. J Clin Endocrinol Metab. 2007; 92: 3197-3201.
- Li M, Sun H, Liu S, Yu J, Li Q, Liu P, et al. CD40 C/T-1 polymorphism plays different roles in GD and HT: a meta-analysis. Endocr J. 2012; 59: 1041-1050.
- Wang D, Chen J, Zhang H, Zhang F, Yang L, Mou Y. Role of different CD40 polymorphisms in Graves disease and Hashimoto’s thyroiditis. Immunol Invest. 2017; 46: 544-551.
- Huber AK, Finkelman FD, Li CW, Concepcion E, Smith E, Jacobson E, et al. Genetically Driven Target Tissue Overexpression of CD40: A Novel Mechanism in Autoimmune Disease. J Immunol. 2012; 189: 3043-3053.
