
Mini Review
Austin Neurol. 2016; 1(2): 1006.
Epigenetics Regulation in Anxiety and Depression
Chaudhury S*
Molecular and Behavioral Neuroscience Institute, University of Michigan, USA
*Corresponding author: Sraboni Chaudhury, Molecular Behavioral Neuroscience Institute, University of Michigan, 205 Zina Pitcher Place, Ann Arbor, MI- 48109, USA
Received: August 22, 2016; Accepted: October 13, 2016; Published: October 19, 2016
Abstract
Stress and anxiety are some of the leading cause various psychiatric illness which shows depression like symptoms. It has been postulated that both genetic components as well as environmental factors are responsible for the etiology of these diseases. With the advancement in the field of epigenetic it has been suggested that epigenetic regulation can act as linkage between gene and environment. Epigenetics is the study of cellular and physiological phenotypic trait differences which regulates various gene expressions during particular condition without altering the nucleotide sequences. Epigenetic regulation includes DNA methylation, histone modification and chromatin modeling, each of which directs gene expression without altering the underlying DNA sequence. In this review, we explore the role of epigenetic regulation in understanding the susceptibility of an individual to stress and anxiety which will help us in developing therapeutic targets for disease related to anxiety and depression.
Keywords: Epigenetics; Stress; Anxiety; Depression
Abbreviations
DNMT: DNA Methyl Transferase; GR: Glucocorticoid Receptor; TE: Transposable Elements; HDAC: Histone Deacetylases
Introduction
Stress and anxiety are primary reason for number of neuropsychiatric disorders. Exposure to early like trauma both during pre and postnatal life as well as during adulthood has a profound effect on various psychiatric illness. With the knowledge of GWAS study number of genes have come up which has shown its relevance in various psychiatric disorder. Role of external factors has shown to affect the pathophysiology of conditions including anxiety and depression. The study showing the relevance of interaction of gene and environment was also established in various psychiatric diseases [1,2]. Epigenetics mechanism, such as DNA methylation and histone protein modifications, and microRNAs function are thought to provide a linkage between the environmental stimuli and gene expression. Epigenetic regulation monitors the transcriptional potential of a cell without altering its DNA sequence. In a complex condition like stress and anxiety there are several genes which has been found to be associated and these genes alter in differential pattern. To further understand the role of various genes, epigenetic regulation associated with genes during early development and in adulthood in stress and anxiety related disorder will provide an insight.
Epigenetic modifications during development
The alteration in epigenetic marks is very important during neurodevelopment and any disruption could lead to significant impacts on neurodevelopment and cognitive function [3,4]. Induction in prenatal stress shows display several abnormalities such as and global changes in DNA methylation including gene promoters in newborn and adult rodents [5]. Adverse fetal and early-life experience with emergent epigenetic changes [6,7] determining lifelong susceptibility to chronic disease states [8,9]. There is evidence which explains that stress, anxiety and depression during pregnancy can influence DNA methylation levels of the glucocorticoid receptor gene in the neonate [10]. Prenatal stress has the capability to alter the expression and DNA methylation status of the corticosteroid metabolic enzyme 11betahydroxysteroid dehydrogenase-2, which results in increased DNA Methyl Transferase (DNMT) 3a expression in placenta and increased DNMT1 expression in the fetal cortex [11]. It is also suggested that the levels of Dnmt3a in medial prefrontal cortex mediates anxiety-like behavior and this may be the primary molecular link between chronic stress and the development of anxiety disorders, including post-traumatic stress disorder [12]. Research suggest that heterochromatin protein 1 binding protein 3 which is epigenetic repressor is important in regulating maternal and anxietyrelated behavior along with offspring survival in mice [13]. Maternal anxiety in pregnancy is associated with decreased DNA methylation associated with imprinted genes, insulin-like growth factor 2 and H19 in progeny at birth, particularly in female with low birth weight neonates [14]. Prenatal alcohol exposure resulting in fetal alcohol syndrome was show to have a distinct DNA methylation patterns in children and adolescents [15]. All these studies suggest that epigenetic regulation has an important role to play in manifestation of anxiety and depression during pre and postnatal development. However the alteration in these epigenetic regulations is not universal and act differentially with different genes. Thus, future studies are required to understand the detail phenomenon/pathway associated with DNA methylation, histone modification and chromatin remodeling in regulating various genes to develop therapeutic targets.
Epigenetic regulation in adults
In the stress literature, histone modifications and DNA methylation have been the most thoroughly examined [16]. Human post-mortem studies have reveal a potential role for Reelin in the etiopathology of diseases such as bipolar, schizophrenic and depressive patients. These patients display low levels of Reelin [17-19]. In stressed adult rats and in schizophrenic patients there is a down-regulation of Reelin through DNA methylation at its promoter sequence [20,21]. Research suggests that histone 3 phospho acetylation have been responsive to stress and anxiety [22-24]. Acute stress using forced swimming has resulted in increased dual phospho-acetyl mark at serine10 and lysine 14 of histone H3 in the rat dentate gyrus [25]. Also histone methylation in the hippocampus is been shown to be associated with stress [26]. It is demonstrated that differences in early life environment which results in long lasting, trans-generational changes in behavior and part of it is mediated by changes in epigenetic histone marks and DNA methylation of the 1-7 promoter of the Glucocorticoid Receptor (GR) in the hippocampus [27]. Further studies have demonstrated that, epigenetic changes in the DNA methylation status of both the GR and Brain derived neurotrophic factor genes could be related with a history of childhood abuse in humans [28,29]. Research haves shown that “junk” DNA, Transposable Elements (TEs) are actively transcribed in brain and environmental stress might modify TEs by via histone modification by both DNA and histone methylation [30]. Social defeat stress, which is one of the better rodent models of human depression [31], also been shown to be modified by epigenetic regulation [32]. It was observed that there is a significant increased the levels of the repressive histone H3K27me3 at the BDNF promoter following chronic social defeat in adult mice, while activated transcription marks, such as H3 acetylation and H3K4 methylation increases after antidepressant drugs treatment [33,34]. Later studies by the same group identified similar changes with alteration in Histone Deacetylase (HDAC) function in the nucleus accumbens following stress or cocaine treatment. Most important, HDAC2 was found to have an antidepressant effect in their same social defeat model [35,36]. Adolescent alcohol exposure in rats alters lysine demethylase 1 expression and repressive Histone Methylation (H3K9me2) in the amygdala during adulthood which show that epigenetic regulation have a role to play in pathophysiology of stress [37]. Several ATPdependent chromatin remodeling factors and Histone Deacetylases (HDACs) was found to be altered in mice with high anxiety trait [38]. Studies on bred lines of high responder and low responder rats which shows characteristics of anxiety- and depression-like behavior shows an epigenetic regulation of genes vulnerable to stress and anxiety [39]. All the above mentioned studies explain that epigenetic regulations are critical not only during development, but also during adulthood. Even in adult individual epigenetic modifications are capable of manipulating stress and anxiety.
Conclusion
Thus, epigenetic modification has a role to play in regulating the conditions related to anxiety and depression. There are number of genes so far have been identified in different brain regions to be associated with stress and anxiety. Further, environmental factors including drugs, alcohol, harmful chemicals and stressful conditions are also have a crucial role to play in the etiology of these disease states. So, it is possible that epigenetic regulation could act as a link between expression of genes and external stimulation (Figure 1). Therefore, future studies should focus on epigenetic factors like DNA methylation, histone modification as well chromatin remodeling to have a better understanding of these psychiatric illnesses. Enzymes associated with various histone modifications are coming up as more promising targets. All this will help us in building more effective drugs which could be further used as coherent therapeutic targets in cure of psychiatric disorder including anxiety and depression.
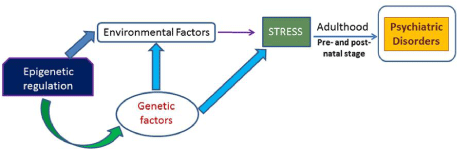
Figure 1: Epigenetic regulation could modulate both environmental factor as
well as genetic component to trigger stress during early developmental stage
or adulthood which may cause psychiatric illness.
References
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006; 7: 583–590.
- Murcray CE, Lewinger JP, Gauderman WJ. Gene-environment interaction in genome-wide association studies. Am J Epidemiol. 2009; 169: 219–226.
- Hutnick LK, Golshani P, Namihira M, Xue Z, Matynia A, Yang XW, et al. DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Hum Mol Genet. 2009; 18: 2875-2888.
- La Salle S, Oakes CC, Neaga OR, Bourc’his D, Bestor TH, Trasler JM. Loss of spermatogonia and wide-spread DNA methylation defects in newborn male mice deficient in DNMT3L. BMC Dev Biol. 2007; 7: 104.
- Mychasiuk R, Ilnytskyy S, Kovalchuk O, Kolb B, Gibb R. Intensity matters: brain, behaviour and the epigenome of prenatally stressed rats. Neuroscience. 2011; 180: 105–110.
- Dammann G, Teschler S, Haag T, Altmüller F, Tuczek F, Dammann RH. Increased DNA methylation of neuropsychiatric genes occurs in borderline personality disorder. Epigenetics. 2011; 6: 1454-1462.
- Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis. 2006; 44: 401–406.
- Waterland RA. Early environmental effects on epigenetic regulation in humans. Epigenetics. 2009; 4: 523–525.
- Spadaro PA, Bredy TW. Emerging role of non-coding RNA in neural plasticity, cognitive function, and neuropsychiatric disorders. Front Genet. 2012; 3: 132.
- Palma-Gudiel H, Cordova-Palomera A, Eixarch E, Deuschle M, Fananas L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: a meta-analysis. Epigenetics. 2015; 10: 893–902.
- Jensen Pena C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11betahydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE. 2012; 7: 3979.
- Elliott E, Manashirov S, Zwang R, Gil S, Tsoory M, Shemesh Y, et al. Dnmt3a in the Medial Prefrontal Cortex Regulates Anxiety-Like Behavior in Adult Mice. J Neurosci. 2016; 36: 730-740.
- Garfinkel BP, Arad S, Neuner S, Netser S, Wagner S, Kaczorowski CC, et al. HP1BP3 expression determines maternal behavior and offspring survival. Genes Brain Behav. 2016; 15: 678-688.
- Mansell T, Novakovic B, Meyer B, Rzehak P, Vuillermin P, Ponsonby AL, et al. The effects of maternal anxiety during pregnancy on IGF2/H19 methylation in cord blood. Transl Psychiatry. 2016; 6: 765.
- Portales-Casamar E, Lussier AA, Jones MJ, MacIsaac JL, Edgar RD, Mah SM, et al. DNA methylation signature of human fetal alcohol spectrum disorder. Epigenetics Chromatin. 2016; 9: 25.
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012; 62: 3-12.
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and Glutamic Acid Decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000; 57: 1061–1069.
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, et al. A Decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA. 1998; 95: 15718–15723.
- Fatemi SH, Earle JA, McMenomy T. Reduction in Reelinimmunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000; 5: 654–663.
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, et al. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005; 134: 60–66.
- Qin L, Tu W, Sun X, Zhang J, Chen Y, Zhao H. Retardation of neurobehavioral development and reelin down-regulation regulated by further DNA methylation in the hippocampus of the rat pups are associated with maternal deprivation. Behav Brain Res. 2011; 217: 142–147.
- Collins A, Hill LE, Chandramohan Y, Whitcomb D, Droste SK, Reul JM. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS One. 2009; 4: 4330.
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007; 53: 857–869.
- Reul JM, Hesketh SA, Collins A, Mecinas MG. Epigenetic mechanisms in the dentate gyrus act as a molecular switch in hippocampus-associated memory formation. Epigenetics. 2009; 4: 434–439.
- Bilang-Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JM. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptordependent behavioural response. Eur J Neurosci. 2005; 22: 1691–1700.
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci USA. 2009; 106: 20912–20917.
- Szyf M, Weaver ICG, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrin. 2005; 26: 139–162.
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009; 12: 342–348.
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of earlylife adversity on the BDNF gene. Biol Psychiatry. 2009; 65: 760–769.
- Hunter RG, McEwen BS, Pfaff DW. Environmental stress and transposon transcription in the mammalian brain. Mob Genet Elements. 2013; 3: 24555.
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010; 13: 1161–1169.
- Renthal W, Nestler EJ. Chromatin regulation in drug addiction and depression. Dialogues Clin Neurosci. 2009; 11: 257-268.
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006; 9: 519–525.
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004; 24: 5603–5610.
- Renthal W, Maze I, Krishnan V, Covington HE, Xiao G, Kumar A, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007; 56: 517–529.
- Covington HE, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009; 29: 11451–11460.
- Kyzar EJ, Zhang H, Sakharkar AJ, Pandey SC. Adolescent alcohol exposure alters Lysine Demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood. Addict Biol. 2016.
- Wille A, Amort T, Singewald N, Sartori SB, Lusser A. Dysregulation of select ATP-dependent chromatin remodeling factors in high trait anxiety. Behav Brain Res. 2016; 311: 141-146.
- Chaudhury S, Aurbach EL, Sharma V, Blandino P, Turner CA, Watson SJ, et al. FGF2 is a target and a trigger of epigenetic mechanisms associated with differences in emotionality: partnership with H3K9me3. Proc Natl Acad Sci USA. 2014; 111: 11834-11839.