
Research Article
Austin Neurol. 2018; 3(1): 1013.
LX9211 A Selective Inhibitor of AAK1 (Adapter- Associated Kinase) for Neuropathic Pain? Some Thoughts on Selectivity and Specificity
Hesselink JMK *
Faculty of Health, University of Witten-Herdecke, Germany
*Corresponding author: Keppel Hesselink JM, Faculty of Health, University of Witten-Herdecke, Germany; Institute for Neuropathic Pain, the Netherlands; Email: jan@neuropathie.nu
Received: April 30, 2018; Accepted: June 08, 2018; Published: June 15, 2018
Abstract
New leads in neuropathic pain anno 2018 are relative rare, compared to other disorders, such as cancer. Lexicon Pharmaceuticals and Bristol- Myers Squibb Company together developed a number of newly synthesized Adapter-Associated Kinase I (AAK1) inhibitors, protected by patents of Lexicon Pharmaceuticals, among which the small molecule LX9211. LX9211 is currently in phase I. AAK1 is a specific kinase, belonging to the Numb-associated family of protein kinases (NAKs). These kinases are known to exert broad biological effects, and AAK1 is instrumental for the invagination of receptor clusters, via clathrin, and thus plays a role in receptor clearance from the membrane. By inhibiting this kinase, receptors belonging to the Gaba-ergic system are thought to be salvaged from clearance and thus AAK1 inhibitors are putative treatments for neuropathic pain. However, the selectivity and specificity of AAK1 inhibitors is still a matter if investigation.
Introduction
It must have been quite a Sisyphus labor, to test 3097 homozygous mouse knockout lines in hot-plate and formalin-paw assays in order to identify novel pain targets. Scientists from Lexicon Pharmaceuticals (LP) and Bristol-Myers Squibb Company (BMS) completed this impressive task and reported its result in 2016 [1]. Their work must have started before 2012, when they first filed patents on results related to these findings. In 2016 they reported their findings in a well-designed paper, including presenting supplemental material in a separate addendum. The paper is an example of how one should address early drug-development issues, and includes dose-finding data and plasma-levels of drugs administered related to their efficacy.
In pharmacology, it is often stated that a drug is highly selective for one receptor only. If so, it is a clean drug, a drug without a promiscuous receptor profile. If a drug has one effect only on biological systems, it is classified as a drug with specificity. Clearly many drugs developed are selective rather than specific [2]. We will have a closer look into the highly innovative approach taken by LP and BMS related to a new target for pain and the development of their lead LX9211, claimed to be a selective inhibitor of AAK1.
In a recent published report, it was pointed out that the industrywide pain pipeline consists of only 125 Novel Chemical Entities (NCE’s) in the clinical phase of development, most of which are related to non-opioid receptors. For comparison, the current pipeline for oncology consists of 1700 NCE’s in the clinical-stage [3]. Nearly all analgesics available (72) belong to one of the following 6 old classes: the NSAIDs, opioids, ionchannel (sodium and calcium) modulators, serotonergic, monoaminergic or gabaergic drugs [3]. In the last decade, only very few new analgesics entered the marketplace, such as tapentadol and milnacipram, both belonging to old classes of drugs. A new class of analgesics therefore would be highly welcome.
A innovative drug, belonging to a new class intended for the treatment of neuropathic pain, LX9211, is currently developed by LP; the drug was previously known as BMS-986176, and is a small molecule. LX9211 was first tested in humans in a phase I study, starting in September 2017 [4]. The special and interesting issue here is that the Adapter-Associated Kinase (AAK) pathway is a totally new target for the treatment of neuropathic pain.
The Adapter-Associated Kinase 1(AAK1)
AAK1 belongs to a series of important kinases of the class of Numb-Associated family of protein Kinases (NAKs), together with amongst others BIKE/BMP2K (BMP-2-Inducible Kinase) and GAK (cyclin G-Associated Kinase). Earlier data already demonstrated that the family of NAKs is able to interact with a wide variety of ligands, and this was stipulated as suggestive for excellent druggability of NAK kinases [5]. Many different kinase inhibitors with excellent selectivity profiles described in the recent past, interacted significantly with members of the NAK family, for instance the JAK inhibitor momelotinib and the FLT3 inhibitor lestaurtinib, probably due to the similarity of the ATP binding sites of the enzymes belonging to the NAK family [5].
The function of the AAK1 pathway as an important regulator of clathrin-mediated endocytosis was first mentioned in 2012 [6]. The endocytosis process is key for the recycling of synaptic vesicles after neurotransmission as well as for receptor-mediated endocytosis. Thus, the AAK1 pathway plays a central role in many biological functions (Figure 1). The key question will be, can we by inhibiting the pathway treat neuropathic pain, without interfering in key biological functions related to the clathrin mediated endocytosis? And will AAK1 inhibitors not lead to off-target adverse events?
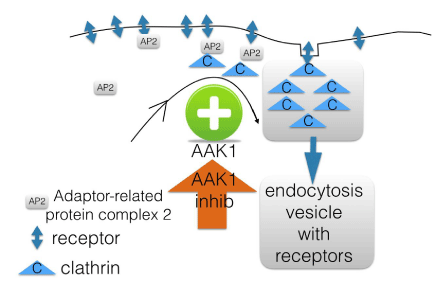
Figure 1: Adaptor-related protein complex 2(AP2)-Associated Kinase 1
(AAK1) stimulates in vagination of vesicles containing receptors; AAK1
inhibitors are targeted for this pathway and keep receptors on membrane.
Screening for knock-outs led to the synthesis of AAK1 inhibitors: patent protected families of molecules BMS and LP screened a great number of knockout mice to identify novel pain targets. Adaptor-related protein complex 2(AP2)-Associated Kinase 1 (AAK1) knockouts were identified based on a reduced response in the screening pain assay. The AP-2 complexes have an important function during receptor-mediated endocytosis to trigger clathrin assembly, relevant for vesicle formation, and interact with a number of membrane-bound receptors (Figure 1).
A Lexicon Pharma patent on AAK1 inhibitors, priority date 9-3-2012, covers the inhibition of AAK1 for the treatment of pain, and fibromyalgia was one of the key indications mentioned [7]. The three inventors of the patent were co-authors of the seminal paper of 2016 [1]. Much of the methods and data mentioned in the paper were described already in the patent of 2012. Other patents of Lexicon covered families of molecules inhibiting AAK1 [8]. In these patents a number of indications were coupled to the families of molecules inhibiting AAK1 activity including Alzheimer’s disease, bipolar disorder, pain, Parkinson’s disease, and schizophrenia, and cognitive deficits in schizophrenia.
After the identification of the AAK1 knock-outs, subsequent synthesis efforts and high-throughput screens led to the identification of a potent and orally available AAK1 inhibitor, with the codename LP-935509 [1].
The working hypothesis put forward in patents and the paper of 2016 is that AAK1 blockers reduce the endocytosis of key cell surface protein(s) involved in transmitting pain signals, especially linked to pain inhibition (supposedly as in the stimulation of the Gaba-ergic pathways). How this works in detail on cellular and sub cellular level remains to be analyzed.
AAK1 related functions and selectivity of inhibitors
There are a number of other AAK1- related functions, such as dendritic branching and length growth, neuritic outgrowth and the regulation of the notch pathway. Furthermore, the AAK1 inhibitors also influence BMP-2-inducible protein kinase (BIKE), relevant in osteoclasts differentiation. LP-935509 was reported to be a potent inhibitor of BIKE and an inhibitor of Cyclin G-Associated Kinase (GAK) as well, both in nanomolar concentrations. This broad unspecific activity on various biological relevant enzyme systems, together with its somewhat unclarified mechanism of action should indicate some early warning flags related to its side-effect profile. One can also question in how far we could or should speak of ‘selective’ inhibition. LX9211 is characterized by the company as a selective inhibitor of AAK1 [9]. However, to date we could not yet identify a paper on the pharmacology of this compound.
Optimization studies showed that AAK1 inhibitors can be synthesized which can penetrate the central nervous system and are orally active. AAK1 inhibitors were effective in various pain models, as well as in both ligation and diabetic neuropathy models. Inhibitors of AAK1 one could further characterize as ‘drugs stilllooking for a disease’, as AAK1 has been suggested to be a putative therapeutic target for the treatment of schizophrenia, cognitive deficits in schizophrenia, Parkinson’s disease, bipolar disorder, and possibly Alzheimer’s disease [10].
Off-target side effects have been reported for NAK family members; for instance, potent inhibition of GAK by the cancer drug gefitinib, an Epidermal Growth Factor Receptor (EGFR) inhibitor, has been linked to respiratory side effects in lung cancer patients [11]. Related to the similar profiles of BIKE compared to AAK1, it has been already suggested that design of selective inhibitors will be quite challenging. JAK inhibitors with reported high selectivity such as for instance momelotinib, baricitinib, ruxolitinib, and gandotinib) have high affinity to NAKs, and as those two kinase families do not have much in common, the extensive overlap in inhibitor activity came as a surprise for the experts. The data presented by Sorrell et al (2015) showing that NAKs can bind with high affinity to many different target inhibitors that were previously reported to be selective, provokes us to be modest in our expectations related to selectivity [6]. Previous experiences with selective FAAH inhibitors, where the specificity remained a question, support such modesty [12]. It therefore remains to be seen in how far the AAK1 inhibitor LX9211 and related molecules will be selective and specific.
References
- Kostich W, Hamman BD, Li YW, Naidu S, Dandapani K, Feng J, et al. Inhibition of AAK1 Kinase as a Novel Therapeutic Approach to Treat Neuropathic Pain. J Pharmacol ExpTher. 2016; 358: 371-386.
- Lounkine E, Keiser MJ, Whitebread S, Mikhailov D, Hamon J, Jenkins JL, et al. Large-scale prediction and testing of drug activity on side-effect targets. Nature 2012; 486: 361-367.
- David Thomas, CFA and Chad Wessel BIO INDUSTRY ANALYSIS The State of Innovation in Highly Prevalent Chronic Diseases. Volume II: Pain and Addiction Therapeutics. 2018.
- Ricotta D, Conner SD, Schmid SL, von Figura K, Honing S. Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol. 2002; 156: 791-795.
- Conner SD, Schmid SL. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol. 2002; 156: 921-929.
- Sorrell FJ, Szklarz M, Abdul Azeez KR, Elkins JM, Knapp S. Family-wide Structural Analysis of Human Numb-Associated Protein Kinases. Structure. 2016; 24: 401-411.
- Lanthorn TH, Savelieva K Zambrowicz B. Inhibition of adaptor associated kinase 1 for the treatment of pain.
- Imidazo [1,2-b] pyridazine-based compounds, compositions comprising them, and methods of their use; Pyrazolo [1,5-a] pyrimidine-based compounds, compositions comprising them, and methods of their use; Inhibitors of adapter associated kinase 1, compositions comprising them, and methods of their use.
- Company communication. Lexicon Pharmaceuticals Initiates Patient Dosing In a Phase 1 Study of LX9211.
- Abdel-Magid AF. Inhibitors of Adaptor-Associated Kinase 1 (AAK1) May Treat Neuropathic Pain, Schizophrenia, Parkinson ’s disease, and Other Disorders. ACS Med. Chem. Lett. 2017; 8: 595−597.
- Tabara H, Naito Y, Ito A, Katsuma A, Sakurai MA, Ohno S, Shimizu H, et al. Neonatal lethality in knockout mice expressing the kinase-dead form of the gefitinib target GAK is caused by pulmonary dysfunction. PLoS One. 2011; 6: e26034.
- Dider S, Ji J, Zhao Z, Xie L. Molecular mechanisms involved in the side effects of fatty acid amide hydrolase inhibitors: a structural phenomics approach to proteome-wide cellular off-target deconvolution and disease association. NPJ SystBiol Appl. 2016; 2: 16023.