
Case Report
Austin Neurol. 2021; 4(1): 1015.
Methylene Tetrahydrofolate Reductase (MTHFR) Mutation in a Young Patient with Stroke: A Case Report
Neethu S¹, Vidya MV² and Abdul Jalal MJ³*
¹Department of Family Medicine, VPS Lakeshore Hospital, India
²Department of Neurology, VPS Lakeshore Hospital, India
³Department of Internal Medicine and Rheumatology, VPS Lakeshore Hospital, India
*Corresponding author: Muhammed Jasim Abdul Jalal, Department of Internal Medicine and Rheumatology, VPS Lakeshore Hospital, Kochi, Kerala, India
Received: January 09, 2021; Accepted: January 27, 2021; Published: February 03, 2021
Abstract
The etiology of young stroke is mainly contributed by genetic mutations in coagulation and metabolic pathways. We present a 29-year-old male, who presented with headache and weakness and later diagnosed to have posterior cerebral artery territory infarct. We highlight MTHFR mutation as an etiology of young stroke and the importance of family screening in such patients.
Keywords: Young stroke; Hyperhomocysteinemia; Methylene tetrahydrofolate reductase (MTHFR); Gene mutation; Headache
Introduction
Cerebrovascular accidents in young are commonly not caused by the usual risk factors in old age rather due to many genetic mutations in coagulation and metabolic pathways. MTHFR C677T polymorphism results in dysregulated folate mechanism and hyperhomocysteinemia. This is a case presented with headache and weakness and later diagnosed to have posterior cerebral artery territory infarct. A search for the etiology revealed hyperhomocysteinemia and associated heterogeneous Methylenetetrahydrofolate Reductase (MTHFR) mutation. Family screening exposed the asymptomatic homocysteinemia in younger brother.
Case Presentation
A 29-year-old male presented with sudden onset vomiting and headache followed by weakness and diminished vision. Patient has history of recurrent migraine attacks with aura. He doesn’t have any seizures or dysarthria or loss of consciousness. General examination didn’t reveal any pallor, icterus, clubbing, cyanosis, and edema or generalized lymph node enlargement. Patient was afebrile with stable vital signs. He was conscious and oriented. Pupils were equal and reactive to light. Extra ocular movements were normal. Cranial nerve examination showed right hemianopia. On motor system examination, upper limb and lower limb had grade 4 powers in right side and grade 5 in left side. There were no sensory losses. All deep tendon reflexes were present. Bilateral plantar reflexes did not show babinski sign. He didn’t have any signs of meningeal irritation or cerebellar signs. Other systemic examinations didn’t reveal any abnormalities.
Baseline blood investigations including Hemogram were within normal limits. Renal and liver function tests, electrolytes and blood sugar values were normal. CT Brain revealed ill-defined hypo-dense area in the left thalamus & posterior limb of internal capsule (Figure 1A) and hypo-dense area in the left parieto-occipital region (Figure 1B). Pro-coagulant and vasculitic work-up were done along with cardiovascular screening and imaging since the patient had stroke in young age. Echocardiogram and holter monitoring were negative for any pathology and vasculitic work up didn’t reveal any positive results. Lipid profile showed dyslipidemia. Homocysteine level was found to be high. Thrombotic profile disclosed heterogeneous mutation of Methylenetetrahydrofolate Reductase (MTHFR) gene. Patient was started on antiplatelet and anti-edema measures with supportive medications. Dyslipidemia was treated with statins. On follow up visit, he had persistent spasticity in right upper limb with improved vision. Family screening for homocystinemia revealed elevated level in his asymptomatic younger brother.
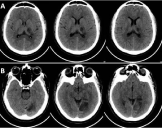
Figure 1: CT Brain showing ill-defined hypo-dense area in the left thalamus &
posterior limb of internal capsule (A) and hypo-dense area in the left parietooccipital
region (B).
Discussion
Central nervous system infarction is defined as brain, spinal cord, or retinal cell death attributable to ischemia, based on neuropathological, neuroimaging, and/or clinical evidence of permanent injury [1]. It is a complex multifactorial polygenic disease and 80-90 % are ischemic in origin. Stroke is usually common among older age group, but young strokes are not uncommon. Risk factors for ischemic stroke are diverse and include hypertension, diabetes, dyslipidemia, low socioeconomic status and family history of stroke. But young people (<40 years) presenting with stroke needs extensive work up to find the cause and initiate appropriate management since most of them are different from usual stroke seen in old age and those are completely amenable to treat too. Our patient was initially treated as complicated migraine from elsewhere since he had a strong history of migraine with aura in the past without being investigated for causes of young stroke.
The causes of stroke in young adults includes extra cranial arterial dissection, cardio embolism, premature atherosclerosis, hematological and immunological disorders, migraine, drug abuse, isolated angitis of CNS, hereditory diseases of connective tissue and other genetically determined disorders [2]. Major etiologies for stroke in young adults were multiple gene mutations rather than systemic illness. The mutation of the MTHFR gene in isolation or in combinations with other gene mutations is the most important risk factors for stroke in young [3].
Despite intensive research efforts genetic etiology of ischemic stroke remains elusive. Hyperhomocysteinemia remains an independent risk factor for atherothrombotic cardiovascular events, stroke, cognitive impairment, and osteoporosis induced bone fracture. Among the genes involved in homocysteine metabolism, Methylenetetrahydrofolate Reductase (MTHFR) C677T gene polymorphism plays a pivotal role by decreasing the activity of MTHFR and increasing homocysteine levels [4]. Elevated homocysteine level is not only risk factor for ischemic stroke but also for other thrombotic and cerebral vascular diseases.
In 2005, Alluri RV et al., conducted a study in Hyderabad with 69 patients of arterial stroke, 6 patients of venous stroke and 49 controls. The prevalence of the mutated homozygous and heterozygous C677T MTHFR genotype in the patients with arterial stroke was 1.4% and 31.88%, respectively [5]. This study indicates that C677T MTHFR mutation is strongly associated with arterial stroke especially in young adults and this allele evaluation helps in prevention and reduction of morbidity caused by stroke [5].
In 2016, Amit Kumar et al., conducted a study in North Indian population with multivariate logistic regression analysis showing independent association between MTHFR C677T gene polymorphism and the risk of ischemic stroke [6]. MTHFRC677T gene polymorphism was found to be independently associated with risk of small vessel disease [6].
In 2016, Rutten-Jacobs LC et al., conducted a study in Europe showing association between MTHFR C677T and lacunar stroke. They also found this gene polymorphism is associated with white matter hyper intensity volume [7].
MTHFR genotype modifies the effects of family history and other risk factors on age at onset of CAD. In T/T homozygotes, family history is associated with earlier onset of CAD, high plasma homocysteine level and stronger association between plasma homocysteine and age at onset of CAD [8]. Randomized control trials have shown that oral supplementation of folic acid, B6 and B12 vitamins substantially lower circulating homocysteinemia levels [9].
In our case, patient was positive for heterogeneous MTHFR mutation and responded well to the appropriate stroke treatment and vitamin supplementation. Family screening in younger brother also showed hyperhomocysteinemia suggestive of hereditary component. Thus, we could provide a primordial prevention of stroke in the family too.
References
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44: 2064-2089.
- Martin PJ, Enevoldson TP, Humphrey PR. Causes of ischaemic stroke in the young. Postgrad Med J. 1997; 73: 8-16.
- Araji AA, Sawaya HR, Sawaya RA. Gene mutations and stroke in the young adult. J Stroke Cerebrovasc Dis. 2014; 23: 2554-2558.
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nature genetics. 1995; 10: 111-113.
- Alluri RV, Mohan V, Komandur S, Chawda K, Chaudhuri JR, Hasan Q. MTHFR C677T gene mutation as a risk factor for arterial stroke: a hospital based study. European journal of neurology. 2005; 12: 40-44.
- Kumar A, Misra S, Hazarika A, Kumar P, Sagar R, Pathak A, et al. Association between Methylenetetrahydrofolate Reductase (MTHFR) C677T gene polymorphism and risk of ischemic stroke in North Indian population: A hospital based case-control study. Egyptian Journal of Medical Human Genetics. 2016; 17: 359-365.
- Rutten-Jacobs LC, Traylor M, Adib-Samii P, Thijs V, Sudlow C, Rothwell PM, et al. Association of MTHFR C677T genotype with ischemic stroke is confined to cerebral small vessel disease subtype. Stroke. 2016; 47: 646-651.
- Mager A, Koren-Morag N, Shohat M, Harell D, Battler A. Family history, plasma homocysteine, and age at onset of symptoms of myocardial ischemia in patients with different methylenetetrahydrofolate reductase genotypes, Am J Cardiol. 2005; 95: 1420-1424.
- Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009; 60: 39-54.