Abstract
Purpose: Choroidal osteoma is a rare tumor which features intraocular bone formation. This tumor can be complicated by choroidal neovascularization which is a cause of impaired vision in this condition. We present a case of choroidal osteoma with choroidal neovascularization as a complication.
Methods: Case report with clinical and angiographic correlation.
Results: A 16 year old female presented with a choroidal osteoma in the left eye complicated by choroidal neovascularization. Intravitreal Anti-Vascular Endothelial Growth Factor (Anti-VEGF) was injected into the left eye. Gradual improvement in vision was noted in the affected eye following the anti-VEGF treatment.
Conclusion: We present a case of choroidal osteoma with choroidal neovascular membranes. Anti-VEGF therapy is employed in the treatment of subfoveal Choroidal Neovascularization (CNV) while photodynamic therapy may be considered for extramacular CNV.
Disclosures: The authors have no financial or proprietary disclosures or conflict of interest with this submission. The views expressed in this submission do not reflect the views, opinions, or policies of the Army or any branch of the Department of Defense.
Keywords: Choroidal osteoma; Choroidal neovascularization; Phthisis bulbi; Anti-Vascular Endothelial Growth Factor
Introduction
Choroidal osteoma is a rare tumor associated with intraocular bone formation. It is often an incidental finding on routine examination but can also present as a cause of decreased vision. We present the case of a young female with a choroidal osteoma as well as brief review of the literature with regards to the evaluation and management of choroidal osteomas.
Case Presentation
A 16 year old female presented to the New England Eye Center with a chief complaint of decreased vision and metamorphopsia in the left eye. The symptoms started three months prior to presentation and were unchanged since they developed. There were no other visual complaints at the time of presentation. She denied any prior ocular history; this was her first eye exam. On review of systems, there was no history of recent or chronic illness such as diabetes mellitus, no steroid use, no recent medication use, no prior eye disease, and no prior history of surgery. The patient also denied any family history of significant vision loss or eye pathology.
Her initial work up began with a comprehensive examination of both eyes. Her visual acuity was 20/20 OD, and 20/25-2 OS. Intraocular pressure was measured as 14 OU. Anterior segment exam was unremarkable in both eyes. In the left eye, Dilated Fundus Examination (DFE) revealed an amelanotic choroidal lesion encompassing the macula with over lying pigment changes at the level of the Retinal Pigmented Epithelium (RPE). Intraretinal Hemorrhage (IRH) and Subretinal Fluid (SRF) were observed in the inferior macula (Figure 1A and Figure 1B).
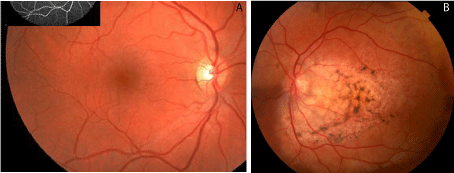
Figure 1: A: Fundus Photo right eye is unremarkable. The inset shows an
unremarkable intravenous fluorescein angiography (IVFA) of the same eye.
B: Yellow/amelanotic Choroidal lesion with overlying retinal pigment
epithelium (RPE) pigment changes encompassing the macula with Intraretinal
Hemorrhage (IRH) and Subretinal Fluid (SRF) of the inferior macula.
Ancillary testing to include Optical Coherence Tomography (OCT), Intravenous Fluorescein Angiography (IVFA), Indocyanine Green (ICG) chorioangiography and B-scan ultrasonography were performed. Intravenous fluorescein angiography was unremarkable in the right eye but showed a pattern of speckled hyperfluorescence in the left eye (Figure 2). ICG Angiography (ICGA) showed early hypofluorescence intermixed with areas of hyperfluorescence (Figure 3). OCT showed distortion of the choroidal anatomy and subretinal fluid or subretinal heme in a juxtafoveal location (Figure 4). Ultrasonography revealed high reflectivity of the lesion with shadowing beyond lesion (Figure 5).
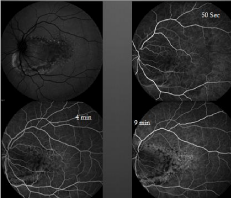
Figure 2: Red free, early, mid, and late phases of Intravenous Fluorescein
Angiography (IVFA) showing spickled hyperfluorescence with late staining in
areas of thinned RPE.
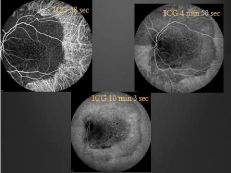
Figure 3: Indocyanine Green (ICG) angiography images showing early
hypofluorescence in areas of the lesion with hyperfluorescence of intralesional
vessels.
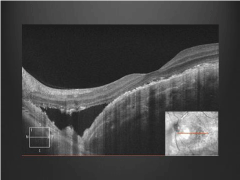
Figure 4: Optical coherence tomography shows distortion of the choroidal
anatomy and Subretinal Fluid (SRF) or Subretinal Heme (SRH).
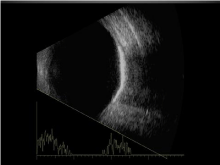
Figure 5: B-scan ultrasonography showing high reflectivity of the lesion with
shadowing beyond lesion.
The differential diagnosis for yellow/amelanotic choroidal lesions is extensive and includes choroidal osteomas, circumscribed choroidal hemangioma (may have overlying fibrous metaplasia, but typically dome-shaped with smooth margins and cystoid changes of the retina, similar A scan, but different IVFA with early hyperfluorescence); Amelanotic Melanoma (more elevation with less well defined margin); Choroidal Metastasis (indistinct margins, often associated with serous RD); Disciform scar in Age-Related Macular Degeneration (ARMD); Sclerochoroidal calcification (wrong age group, occur outside retinal vascular arcades near equator, not juxtapapillary); and Linear nevus sebaceous syndrome (appearance of calcification within the choroid but no bone formation). This is not an all-inclusive list but includes some of the other conditions considered.
Two months after her initial presentation, the patient presented with a complaint of increasing loss of vision and worsening metamorphopsia in the left eye. These symptoms were causing difficulty with reading. Her visual acuity was now 20/40-1 in the left eye. Repeat DFE showed increased subretinal fluid (SRF). A repeat IVFA was performed (Figure 6) and was concerning for increased leakage compared to the prior exam. Optical coherence tomography angiography (OCTA) was performed and showed a choroidal neovascular membrane (Figure 7 and Figure 8).
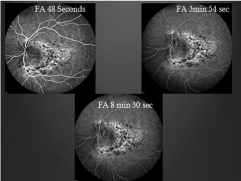
Figure 6: Repeat intravenous fluorescein angiography; early
hyperfluorescence in areas of thinned RPE and late staining–increased
hyperfluorescence compared to her prior study (Figure 2).
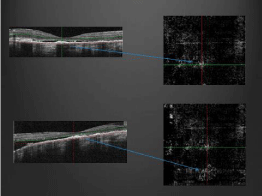
Figure 7: Optical Coherence Tomography Angiography (OCTA) showing
choroidal neovascular membrane and corresponding location on OCT.
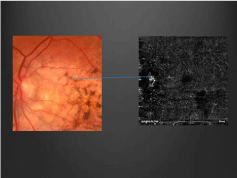
Figure 8: Pathognomonic feature: feeder vessels/vascular spiders located on
tumor surface–vessels that exit holes of cancellous bone of tumor.
Given the presence of a choroidal neovascular membrane, the decision was made to treat right away with intravitreal Anti-Vascular Endothelial Growth Factor (Anti-VEGF). The plan was to give three initial monthly anti-VEGF injections and then as needed depending on her response. The first treatment was given and the patient was scheduled to follow up in a month with plans for a second treatment. At the one month follow up visit, she had gained one line of vision and was now 20/30. There was also new area of IRH noted on the inferior aspect of the lesion. The second injection of intravitreal anti- VEGF was given then with follow up scheduled for 1 month out.
Discussion
Choroidal osteomas are predominately found in the juxtapapillary and macular region of young women (90%), in the second and third decades of life [1]. They are typically found in otherwise healthy eyes in sharp contrast to other eyes in which bone formation may be found: Phthisis bulbi and cyclitic membranes. They generally occur sporadically with only a few cases of familial choroidal osteomas in the literature [2]. Choroidal osteomas are unilateral 75% of the time [1]. They may be asymptomatic, or may cause visual symptoms such as blurring of vision, metamorphopsia, or visual field defects corresponding to the tumor location [1]. Tumor growth was reported in 41% of 22 cases over 10 years [3] and size ranges have been reported as anywhere from 2-22mm in basal dimension and 0.5 -2.5mm in elevation [3]. Decalcification of the tumor may occur spontaneously or may be induced by laser treatment and the rate of partial or complete decalcification is reported as 28% of choroidal osteomas by 5 years and 46% at 10 years [4].
Choroidal Neovascularization (CNV) has been noted with choroidal osteomas and is a known cause of late decreased vision [1]. The typical presentation is a complaint of new metamorphopsia or scotoma and a finding of serous or hemorrhagic detachment of the macula is not uncommon. One study found a CNV rate of 22% at 5 years and 51% at 10 years [5] while another study reported a rate of 31% at 5 years, 47% at 10 years and 56% at 20 years [3]. The exact etiology or pathogenesis of CNV in choroidal osteomas remains unclear.
The diagnostic approach is usually a combination of complete ophthalmic clinical exams and ancillary testing to include IVFA, ICG, and B-scan. Other imaging modalities such CT scans may be employed to confirm findings such as plate-like thickening of the eye wall. Some of the classic findings associated with ancillary testing include early patchy or speckled hyperfluorescence with late staining seen on IVFA (Figure 3). With ICGA, there is hypofluorescence with hyperfluorescence of intralesional vessels. Late leakage on ICG is suggestive of CNV. B-scan ultrasonography typically shows high reflectivity, with orbital shadowing beyond the lesion as seen in (Figure 5). Histopathology reveals plaques of dense bony trabeculae with endothelial lined large cavernous spaces and small capillary blood vessels in the choroid [1].
CNV associated with choroidal osteomas is often an indication for treatment due in part to the high association of CNV with decreased vision. Reasons for decreased vision in CNV include SRF, Subretinal Heme (SRH), RPE atrophy and/or photoreceptor atrophy. Subfoveal CNV is usually treated with anti-VEGF agents and extrafoveal CNV may be approached with Photodynamic Therapy (PDT) or laser photocoagulation. Decalcified osteomas have been reported not to grow in the direction of decalcification; extrafoveal osteomas have been shown to undergo decalcification within1 year of treatment with PDT [4].
Choroidal osteoma is well studied rare tumor of the choroid. It is considered a benign tumor of the choroid which may be asymptomatic or may lead to impaired vision in certain cases. The approach to evaluation and management of choroidal osteomas is largely unchanged with the most recent changes being the use anti- VEGF for management of subfoveal CNV and recently, OCTA is now being employed for the detection CNV. The patient in our case noted a slight improvement with treatment without progression of the lesion and continues to be followed.
References
- Shields CL, Shields JA, Augsburger JJ. Choroidal Osteoma. SurvOphthaimol. 1988; 33: 17-27.
- Noble KG. Bilateral choroidal osteoma in three siblings. Am J Ophthalmol. 1990; 109: 656-660.
- Aylward GW, Chang TS, Pautler SE, Gass JD. A long-term follow-up of choroidal osteoma. Arch Ophthalmol. 1988; 116: 1337-1341.
- Trimble SN, Schatz H. Decalcification of a choroidal osteoma. Br J Ophthalmol. 1991; 75: 61–63.
- Shields CL, Sun H, Demirci H, Shields JA. Factors predictive of tumor growth, tumor decalcification, choroidal neovascularization, and visual outcome in 74 eyes with choroidal osteoma. Arch Ophthalmol. 2005; 123: 1658-1666.
