Abstract
We have previously shown that the retinal rod Outer Segments (OS), although devoid of mitochondria, express functional FoF1-ATP synthase and conduct oxidative phosphorylation. Similar to its mitochondrial counterpart, the rod OS ectopic FoF1-ATP synthase is inhibited by polyphenolic compounds. Grape, one of the first crops domesticated by humans, is currently used for a number of dietary products worldwide. As grape extract contains a mixture of polyphenols, we studied its effects on the OS ATP synthetic activity. ATP synthesis analysis was performed by spectrophotometry. Results demonstrated that commercial grape extract inhibits the OS ATP synthesis up to 98 % in a dose-dependent manner (final concentrations ranging from 0 to 500μg/ml). Presumably due to its elevated content in polyphenolic phytochemicals, grape extract can modulate the OS ATP synthase and subsequently lower the reactive oxygen species production by the ectopic respiratory chain coupled to FoF1- ATP synthase. Further studies may shed light on the molecular mechanism underlying the well-known beneficial effect of grapes and their extracts on the visual system; this could be beneficial in ocular conditions caused by oxidative stress such as age-related macular degeneration and diabetic retinopathy.
Keywords: Grape extract; F1Fo-ATP synthase; Quercetin; Resveratrol; Rod outer segment
Abbreviations
AMD: Age-Related Macular Degeneration; ATP Synthase; F1Fo-ATP Synthase; DR: Diabetic Retinopathy; ETC: Electron Transport Chain; OS: Rod Outer Segment; OXPHOS: Oxidative Phosphorylation; PUFA: Polyunsaturated Fatty Acids; ROI: Reactive Oxygen Intermediates; SD: Standard Deviation.
Introduction
Polyphenols are secondary metabolites widely distributed in plants, where they play metabolic roles and protect plants against UV, pathogen and herbivores [1]. Polyphenols include a wide variety of molecules with structural phenolic features that can be chemically classified according to the number of phenolic rings, and the substituents. The two main groups are the flavonoids, encompassing six major subgroups, and the non-flavonoids, comprising stilbenes [2]. Flavonoids, responsible for the colors of the flowers and fruits, are the most abundant polyphenols in human diet and widely studied. More than 8,000 different flavonoids have been identified. Phenolic compounds, especially anthocyanins, are abundant in Grape (Vitis spp.) [1]. These, abundantly included in the human diet, could alleviate the oxidative stress [3,4] by virtue of their ability to modulate several cellular processes such as proliferation, apoptosis and redox balance. Fruits like grapes and berries contain up to 300mg polyphenols per 100grams fresh weight [1]. Also resveratrol (a stilbene) is largely found in grape [5]. The complex variety of compounds present in grape has demonstrated to possess therapeutic properties. Recent studies have shown that the beneficial health effects promoted by grape can be attributed to its unique mix of polyphenolic compounds [3]. Epidemiological studies have shown that the consumption of grape and grape products lowers the risk of myocardial infarction [6]. Polyphenol intake seems to also act as neuroprotectant [7]. Oxidative stress is a major player in the pathogenesis of retinal degenerative diseases, such as Age Related Macular Degeneration (AMD) and Diabetic Retinopathy (DR) [8,9]. Photoreceptors consume about 4-folds more oxygen than the other retinal cells [10], indicating an active role of the rod OS in the O2 consumption of the outer retina [11], consistently to our previous proteomic and biochemical data reporting the ectopic presence and activity of FoF1-ATP synthase (ATP synthase) and the four respiratory chain complexes and cytochrome c in isolated rod OS [12–14]. It was demonstrated that the retinal rod Outer Segment (OS) more than the inner segment is the target of the oxidative stress-related cytotoxicity caused by exposure of mouse eyes to blue light [15,16]. We have also shown that the blue light-induced detrimental effects cause an impairment of the extra-mitochondrial oxidative phosphorylation in the rod OS, as a consequence of the oxidative damage [17], and that resveratrol, curcumin, quercetin and epigallocatechin gallate exert an inhibitory effect on both the ATPase and ATP synthase rod OS activity [18], consistent with the hypothesis that the OS express a functional ATP synthase [12]. This sheds light on the beneficial effect polyphenolic compounds exert on many retinal pathologies such as age related macular degeneration and diabetic retinopathy [4,7,19]. Here we tested the effect of a natural red grape extract on bovine purified rod OS.
Materials and Methods
Materials
All reagents were of analytical grade. MilliQ (Merck-Millipore) water was utilized throughout. Commercial red grape extract from a local Italian producer was utilized.
Extraction of retinas
Retinas were extracted from freshly enucleated bovine eyes (obtained from a local slaughterhouse) by a procedure we had developed [20] maximizing ROS yield. Briefly, eyecups deprived of vitreous and lens, are filled with Mammalian Ringer (0.157 MNaCl, 5 mMKCl, 7 mM Na2HPO4, 8 mM NaH2PO4, 0.5 mM MgCl2, 2 mM CaCl2 pH 6.9 plus protease inhibitor cocktail (Sigma-Aldrich, S. Louis, MO, USA) and 50μg/ml Ampicillin, for 10min. Then floating retinas are cut free of the optic nerve.
Purified bovine rod OS preparations
Purified bovine rod OS were prepared under dim red light from 20 retinas at 4°C, by sucrose/Ficoll continuous gradient centrifugation [18,21] in the presence of protease inhibitor cocktail (Sigma–Aldrich, S. Louis, MO) and ampicillin (50μg/ml). Rod OS preparation was routinely characterized for integrity of plasma membrane. ROS homogenates were obtained by mini glass-glass Potter homogenize on ice in 4:1 (w/v) hypotonic medium (5 mMTris-HCl pH 7.4, in MilliQ water, plus protease inhibitor cocktail and 50μg/ml ampicillin).
ATP synthesis assay in OS homogenates
The formation of ATP from ADP and inorganic phosphate was performed in rod OS according to our previous report [12]. Rod OS (0.04μg protein/ml) were incubated for 5 min at 37°C in 50mM Tris/ HCl (pH 7.4), 5mM KCl, 1mM EGTA, 5mM MgCl2, 0.6mM ouabain, 0.25mM di(adenosine)-5-penta-phosphate(Ap5A, adenylate kinase inhibitor), and 25μg/ml ampicillin. ATP synthesis was induced by adding 5 mM KH2PO4, 20mM succinate, 0.35mM NADH, and 0.3mM ADP at the same pH of the mixture. After stopping the reaction with 7% perchloric acid final concentration, the ATP concentration in each sample was measured using a spectrophotometrical method. Neutralized and clarified supernatant was added to a mixture containing 2mM MgCl2, 0.5mM NADP, 5mM Glucose, 100mM Tris/HCl pH 7.4 and 7U/ml of a mix of hexokinase and glucose-6- phosphate dehydrogenase (Roche Diagnostics Corp., Indianapolis, IN). NADP+ reduction was followed at 340nm using a dual-beam spectrophotometer (UNICAM UV2, Analytical S.n.c., Italy). Where necessary incubation medium contained 10μMoligomycin, 30μM resveratrol, 100μM quercetin or grape extract with final concentration between 1,25 to 500μg/ml.
Results
Purified rod OS synthesize ATP, through the ectopic expression of the mitochondrial ATP synthase in the disk membranes [12-14]. Considering that the OS ATP synthase is inhibited by polyphenolic phytochemicals [18] (such as resveratrol and quercetin, both abundant in grapes), here we tested a red grape crude extract. The purified bovine rod OS were previously extensively characterized, excluding contamination by mitochondria and IS organelle.ATP synthesis by OS homogenates is reported (Figure 1). A maximal activity of 0,5±0,03μmol/min/mg of protein was detected in the presence of 0.35m MNADH, 20mM succinate and 0.3mM ADP. ATP synthesis was specific, as shown by its inhibition by oligomycin (90%) and resveratrol (98%), inhibitors of Fo and F1 moiety respectively (Figure 1). Moreover, ATP production was inhibited by quercetin (85%), a phenolic compound abundant in grape extract together with resveratrol. Grape extract inhibited ATP synthesis in doseddependent manner by 9, 6% 41, 74, 91, 85, 88 and 100%. At the final concentrations 1.5, 2.5, 5, 10, 20, 125, 250, 500 μg /ml, respectively (Figure 2).
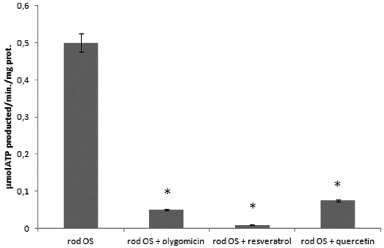
Figure 1: ATP synthesis in purified OS with oligomycin, resveratrol and
quercetin. Histogram shows ATP formation over1min, at 37°C, at pH 7.3
by rod OS (0.04mg/ml). Addition of 10μMoligomycin, 30μM resveratrol or
quercetin inhibited ATP production by 90%, 98% and 85% respectively. Each
point, representative of four separate experiments, is the mean±SD; paired
Student’s t test was used. *p < 0.01.
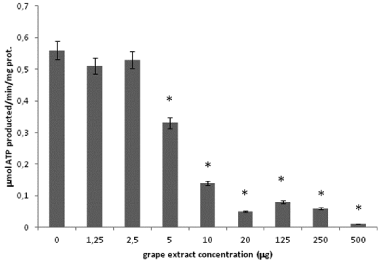
Figure 2: ATP synthesis in purified OS with grape extract. Histogram
shows ATP formation over1min, at 37°C, at pH 7.3 by OS (0.04mg/ml).
Addition of 1.5, 2.5, 5, 10, 20, 125, 250 or 500μg/ml grape extract inhibited
ATP synthesis by 9, 6% 41, 74, 91, 85, 88 and 100%, respectively. Each
point, representative of four separate experiments, is the mean±S.D; paired
Student’s t test was used. *p < 0.01.
Discussion
Here we have shown that a natural red grape extract can inhibit ATP synthesis by retinal rod OS homogenates in a dose dependent manner (Figure 2).The proven health benefits of Grapes appear related to its content in phytochemicals [22]. Grape extracts contains five major phenolic compounds: catechin and epicatechin in seeds, and quercetin, rutin and resveratrol in skin extracts [3,5,23]. Considering that resveratrol and quercetin are major components of red grape extract, data appear confirmative of our previous report [18] showing that these inhibit the ectopic rod OS ATP synthase. Consistently, the effect of resveratrol and quercetin as single molecules appears to compare with the effect obtained utilizing the grape extract as a whole (Figure 1 and Figure 2).
Beneficial effect of polyphenols on retinal diseases
Vitis vinifera (Black grapes) is traditionally used not only as a food but also as a medicament. Evidence for a beneficial role of antioxidants, especially of natural polyphenolic compounds on the eye and retinal diseases is accumulating [4,24]. Cells exposed to oxidative stress undergo oxidative damage, related to the onset of retinal degenerative pathologies. Flavonoids are characterized by high antioxidant properties. In the case of grapes, it was shown that its antioxidant activity is correlated with its total phenolic contents [22]. Botanical compounds were shown to prevent vision threatening eye diseases such as Age-Related Macular Degeneration (AMD) and DR [25]. The use of antioxidant therapies reduced the Reactive Oxygen Intermediates (ROI) burden in AMD, an oxidative-stress related retinopathy [8]. Grapes contain considerable quantities of resveratrol, and quercetin, which play protective role on the retina [5,26]. Quercetin is one of the most widely studied flavonoids, with protective effects through inhibition of proinflammatory molecules as well as direct inhibition of the intrinsic apoptosis pathway [27]. It was observed that quercetin can prevent the decrease in mitochondrial function due to exposure to hydrogen peroxide and inhibit the production of reactive oxygen intermediates, reducing oxidative cellular damage [28,29]. In vivo experiments reported a diminished choroidal retinal angiogenesis characteristic of AMD by quercetin treatment [30]. Resveratrol extracted by grape wine reduced diabetes-induced vascular lesions, vascular endothelial growth factor production and oxidative stress in animal models [26]. Polyphenols are also beneficial for vascular dysfunction in the DR [31]. In fact and the pathogenesis of the major blinding diseases of the western world, such as AMD, DR and glaucoma, involves oxidative stress-mediated photoreceptor cell death. Probably the cell loss in several disorders of the retina, including Retinitis Pigmentosa (RP), glaucoma, and AMD, is caused by the particular sensitivity of photoreceptors to oxidative stress [32].
Light-exposure damage
It was shown that exposure to 3000 lux of light for up to 120min caused photoreceptor and pigment epithelium (RPE) apoptosis in albino rats, with a preferential vulnerability of rods over cones [33]. Moreover, the rod apoptosis was promptly induced within 90minutes of light exposure, while the onset of RPE apoptosis showed a delay of several hours [33]. This is consistent with the data showing that presence of an extra-mitochondrial oxidative phosphorylation in the rod OS, due to the expression of respiratory complexes I to IV and ATP synthase, that can better explain the pathogenesis of retinal degenerations ascribed to oxidative stress [19]. In mouse eyeculture model of blue-light induced retinal damage the main target of oxidation was the OS of the retinal rod. We have shown that as a consequence of the ROI generation, the extra mitochondrial oxidative phosphorylation in the OS is severely impaired. In particular when photo transduction is persistent activated as during continuous illumination, a faster functioning of the respiratory complexes produces more ROI, triggering a caspase-9 and -3 dependent cell death in response to the release of cytochrome c from the peroxidised disk membranes [34]. This would offer a better explanation of the mechanism by which light exposure the cause’s apoptosis of the rod.
Therefore, it seems that the classical view according to which the oxidative damage starts in the RPE, in a retinal damage [35] should be reconsidered.
Molecular targets of polyphenols
Antioxidants act either directly by counteracting oxidative stress through scavenging free radicals, or oxidation reactions, or indirectly, by up-regulating cellular antioxidant defenses or inhibiting pro-oxidative enzymes [36]. ATP synthase consists of two functional domains: F1, a water-soluble catalytic complex, and Fo, which contributes to the stalk [37]. Sir J. Walker had shown that both resveratrol and quercetin can insert in the F1 head of the nanomotor, hindering its rotary catalysis [38]. By contrast, these are unable to permeate the mitochondrion [39]. In this respect, the molecular targets of polyphenols may be reconsidered. As far as the action of polyphenols on the retinal rod OS is concerned, their antioxidant activity would be indirect. Their interaction with the ectopic ATP synthase, inhibiting its rotary catalysis, would modulate the ectopic respiratory chain, a major source of ROI: it was shown that ROI are produced especially by one of the Flavin Mononucleotides (FMN) groups of the electron transfer chain Complex I [40]. When the respiring membrane is coupled, the activity of ATP synthase is the rate limiting process [37,41]: it can be supposed that any compound able to reversibly modulate its rotary catalysis would limit the ROI production by the electron transfer chain. The ability to reduce ROI production in the rod OS may not be a minor mode of action of polyphenols on the retina, and could offer a scientific validation for their potential use in the prevention or therapy of degenerative retinopathies, particularly in the form of natural source compounds, such as grape extracts.
References
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009; 2: 270-278.
- Falcone Ferreyra ML, Rius SP, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012; 3: 222.
- Georgiev V, Ananga A, Tsolova V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients. 2014; 6: 391-415.
- Qureshi AA, Khan DA, Mahjabeen W, Papasian CJ, Qureshi N. Suppression of Nitric Oxide Production and Cardiovascular Risk Factors in Healthy Seniors and Hypercholesterolemic Subjects by a Combination of Polyphenols and Vitamins. J Clin Exp Cardiolog. 2012; 5: 8.
- King RE, Bomser JA, Min DB. Comprehensive Reviews in Food Science and Food Safety Bioactivity of Resveratrol. Compr Rev Food Sci Food Saf. 2006; 5: 65.
- Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005; 45: 287-306.
- Lee HS, Jun J-H, Jung E-H, Koo BA, Kim YS. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules. 2014; 19: 12150-12172.
- Khandhadia S, Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expert Rev Mol Med. 2010; 12: 34.
- Testa R, Bonfigli AR, Genovese S, De Nigris V, Ceriello A. The Possible Role of Flavonoids in the Prevention of Diabetic Complications. Nutrients. 2016; 8.
- Hoang QV, Linsenmeier RA, Chung CK, Curcio CA. Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation. Vis Neurosci. 2002; 19: 395-407.
- Stefansson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol n.d. 2006; 51: 364-380.
- Panfoli I, Calzia D, Bianchini P, Ravera S, Diaspro A, Candiano G, et al. Evidence for aerobic metabolism in retinal rod outer segment disks. Int J Biochem Cell Biol. 2009; 41: 2555-2565.
- Panfoli I, Calzia D, Ravera S, Bruschi M, Tacchetti C, Candiani S, et al. Extramitochondrial tricarboxylic acid cycle in retinal rod outer segments. Biochimie. 2011; 93: 1565-1575.
- Calzia D, Barabino S, Bianchini P, Garbarino G, Oneto M, Caicci F, et al. New findings in ATP supply in rod outer segments: insights for retinopathies. Biol Cell. 2013; 105: 345-358.
- Roehlecke C, Schumann U, Ader M, Knels L, Funk RH. Influence of blue light on photoreceptors in a live retinal explant system. Mol Vis. 2011; 17: 876-884.
- Roehlecke C, Schumann U, Ader M, Brunssen C, Bramke S, Morawietz H, et al. Stress reaction in outer segments of photoreceptors after blue light irradiation. PLoS One. 2013; 8: 71570.
- Calzia D, Panfoli I, Heinig N, Schumann U, Ader M, Traverso CE, et al. Impairment of extramitochondrial oxidative phosphorylation in mouse rod outer segments by blue light irradiation. Biochimie. 2016; 125: 171-178.
- Calzia D, Oneto M, Caicci F, Bianchini P, Ravera S, Bartolucci M, et al. Effect of polyphenolic phytochemicals on ectopic oxidative phosphorylation in rod outer segments of bovine retina. Br J Pharmacol. 2015; 172: 3890-3903.
- Panfoli I, Calzia D, Ravera S, Morelli AM, Traverso CE. Extra-mitochondrial aerobic metabolism in retinal rod outer segments: New perspectives in retinopathies. Med Hypotheses. 2012; 78: 423-427.
- Bianchini P, Calzia D, Ravera S, Candiano G, Bachi A, Morelli A, et al. Live imaging of mammalian retina: rod outer segments are stained by conventional mitochondrial dyes. J Biomed Opt. 2008; 13: 54017.
- Schnetkamp PP, Daemen FJ. Isolation and characterization of osmotically sealed bovine rod outer segments. Methods Enzym. 1982; 81: 110-116.
- Liang Z, Cheng L, Zhong G-Y, Liu RH. Antioxidant and Antiproliferative Activities of Twenty-Four Vitis vinifera Grapes. PLoS One. 2014; 9: 105146.
- Iacopini P, Baldi M, Storchi P, Sebastiani L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J Food Compos Anal. 2008; 21: 589-598.
- Panfoli I. Beneficial effect of antioxidants in retinopathies: a new hypothesis. Med Hypothesis, Discov Innov Ophthalmol. 2012; 1: 76-79.
- Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Asp Med. 2006; 27: 1-93.
- Kim WT, Suh ES. Retinal protective effects of resveratrol via modulation of nitric oxide synthase on oxygen-induced retinopathy. Korean J Ophthalmol. 2010; 24: 108-118.
- Cao X, Liu M, Tuo J, Shen D, Chan C-C. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp Eye Res. 2010; 91: 15-25.
- Kook D, Wolf AH, Yu AL, Neubauer AS, Priglinger SG, Kampik A, et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 2008; 49: 1712-1720.
- Areias FM, Rego AC, Oliveira CR, Seabra RM. Antioxidant effect of flavonoids after ascorbate/Fe(2+)-induced oxidative stress in cultured retinal cells. Biochem Pharmacol. 2001; 62: 111-118.
- Zhuang P, Shen Y, Lin BQ, Zhang WY, Chiou GC. Effect of quercetin on formation of Choroidal Neovascularization (CNV) in Age-Related Macular Degeneration(AMD). Eye Sci. 2011; 26: 23-29.
- Costa BL, Fawcett R, Li GY, Safa R, Osborne NN. Orally administered epigallocatechin gallate attenuates light-induced photoreceptor damage. Brain Res Bull. 2008; 76: 412-423.
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003; 76: 397-403.
- Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye. 2001; 15: 376-383.
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999; 144: 281-292.
- Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996; 37: 1236-1249.
- Czerska M, Mikolajewska K, Zielinski M, Gromadzinska J, Wasowicz W. Today’s oxidative stress markers. Med Pr. 2015; 66: 393-405.
- Boyer PD. The ATP synthase--a splendid molecular machine. Annu Rev Biochem. 1997; 66: 717-749.
- Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci USA. 2007; 104: 13632-13637.
- Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000; 130: 1115-1123.
- Lenaz G, Genova ML. Supramolecular organisation of the mitochondrial respiratory chain: a new challenge for the mechanism and control of oxidative phosphorylation. Adv Exp Med Biol. 2012; 748: 107-144.
- Boyer PD. Energy, Life, and ATP (Nobel Lecture). Angew Chemie Int Ed. 1998; 37: 2296-2307.
