Introduction
Diabetic retinopathy is a frequent and blinding pathology. Optical Coherence Tomography Angiography (OCTA) could be a promising technique for detecting DR. The aim of our work was to study the interest of OCTA in the detection of microvascular anomalies in diabetics without DR.
Patients and Methods
This was a prospective study of 49 patients (40 eyes) in Ophthalmology Department B at the Rabat Specialty Hospital. 28 patients (24 eyes) were diabetic without diabetic retinopathy (DRS) and 21 patients (16 eyes) were Non-Diabetic (ND).
The parameters studied were the size and remodeling of the Central Avascular Zone (CAZ), the presence of microaneurysms, non-perfusion zones, vascular tortuosity and vascular density in the superficial plexus.
Results
The mean surface area of the CAZ was 0.38 ± 0.1 mm2 in the SRD group versus 0.28 ± 0.1 mm2 in the ND group (p=0.023).
The mean values for parafoveal and total vascular density were 18.77 ± 1.68mm-1 and 18.47 ± 2.94mm-1 respectively in the SRD group versus 20.20± 2.92mm-1 and 20.45 ± 3.36mm-1 in the ND group (p= 0.082 and 0.011 respectively). ZAC remodeling and micro-aneurysms were significantly higher in the SRD D group (p<10-4) Vascular tortuosity was noted in 33.3% of SRD diabetics and in 18.75% of ND subjects (p=0.040), non-perfusion zones were present in 83.3% of SRD diabetics and in 37.5% of ND subjects (p=0.013).
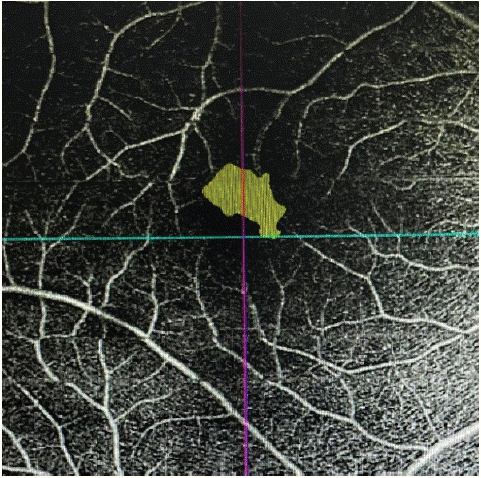
Figure 1: Measurement of the surface area of the ZAC of the superficial plexus on a 6 × 6 mm angiogram in a male.
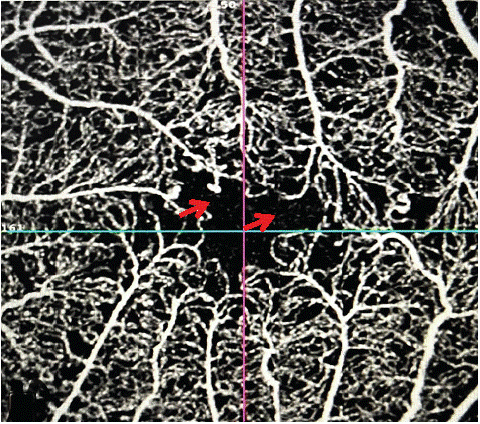
Figure 2: Remodeling of the ZAC in a diabetic patient with rupture of the anastomotic arch (red arrow) on a 3x3 angiogram.
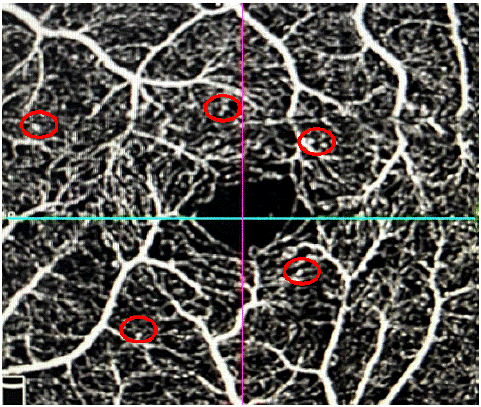
Figure 3: Presence of micro-aneurysms in a diabetic patient on a 3 × 3 mm angiogram.
Discussion
At the end of this study, we concluded that OCTA can detect these microvascular anomalies at a sub-clinical stage by identifying qualitative changes in the retinal microcirculation, such as micro-aneurysms and remodelling of the ZAC.
Indeed, micro-aneurysms are the first clinically detectable signs of DR [1], representing indirect signs of retinal ischemia.
In our series, they were present in 70.8% of SRD diabetics and absent in healthy subjects. Cao et al. found micro-aneurysms in 11.3% of SRD diabetic eyes, but proposed to classify them as "preclinical DR [2], De Carlo et al. found micro-aneurysms in 8% of SRD diabetics [3], and in Vujosevic's series they were more frequent in both plexi for type 1 diabetics and only in the deep plexus for type 2 diabetics in the SRD diabetic group. In the series by Goudotet al, they were absent [4].
As for remodelling of the ZAC, in 2011, using adaptive optical scanning laser ophthalmoscopy, Tam et al. were able to show an alteration in the peri-foveal capillary network in type 2 diabetic subjects without DR [5].
Over time, A-OCT has established itself in this field of research, and several authors have been able to demonstrate more frequent remodeling of the ZAC in diabetic subjects without DR than in normal subjects.
Among these authors, De Carlo et al. defined ZAC remodeling as the presence of adjacent ZNPs, ZAC asymmetry or loss of normal perifoveal capillary spider-web architecture, and the frequency of these lesions in diabetic subjects without DR led them to conclude that these irregularities may be the first signs of DR [3].
Subsequently Vujosevic, Cao and Choi came to the same conclusion [2,6,7].
In our series remodeling of the ZAC a type of ZNPs, tortuosities, ruptures and irregularities of the capillary border was more frequent in SRD diabetic subjects than in normal subjects, with a statistically significant difference (P<104).
We believe that remodeling would be a more valuable argument for microvascular modification than the calculation of ZAC area, as it is less subject to inter-individual variability.
However, the area of capillary ischemia, vascular density, areas of non-perfusion and vascular tortuosity are not sensitive markers of early RD in our series.
The mean ZAC area was larger in the SRD group without there being a statistically significant difference in our series. Cao, Mastropasqua and Nesper found no significant difference between the two groups in their studies and attributed this to the large inter-individual variability of ZAC area in healthy subjects, and concluded that ZAC area is not a sensitive marker for defining early DR [2,8,9], in agreement with the studies by Simonett and Carnevali, who explained this by the characteristics of the population studied, namely the young age of the patients, the type of diabetes (type1) and a lower incidence of associated pathologies such as hypertension and dyslipidemia than in the other studies [10,11].
Goudot et al. calculated a ZAC widening coefficient between the superficial and deep plexus in search of early microvascular anomalies, independently of inter-individual ZAC variability, and found no significant difference between the two groups [4]. Dimitrova, De Carlo and Li found a widening of the ZAC in the deep and superficial plexus, with a significant difference only in the superficial plexus; they explained this by the reduction in parafoveal vascular density [3,12,13]. Takase et al. found a widening of the ZAC in the deep plexus in SRD diabetics and concluded that damage to the macular microcirculation precedes the development of DR [14]. In more recent studies, Vujosevic, Alibhai and Kim have shown an enlargement of the ZAC in both plexi in SRD diabetics.
With regard to vascular density, most studies have focused on both the deep and superficial plexus [15]. Alibhai, Goudot and Mastropasqua studied parafoveal vascular density in both plexi and found no significant difference between the two groups [4,8,16].
Simonett and Carnevali, in their studies of type 1 diabetes, showed a decrease in parafoveal capillary density in the deep plexus, but none in the superficial plexus, and concluded that this factor is an early sign of DR in type 1 diabetics, even preceding the widening and remodelling of the ZAC [10,11]. Studies by Dimitrova and Cao showed a significant decrease in perfoveal vascular density in both the deep and superficial plexus between the SRD diabetic group and the non-diabetic group [2,12]. In their study, Li et al. demonstrated a significant decrease in total and parafoveal capillary density in both plexi in type 2 diabetic subjects compared with healthy subjects [13].
In our study, we were unable to demonstrate a significant decrease in total and parafoveal capillary density in the superficial plexus between the two groups. Previous studies using FA techniques have already demonstrated the presence of ZNPs in diabetic patients SRD Consequently, ZNPs are another microvascular change that may precede any abnormality occurring in diabetic eyes and can now be easily assessed by OCTA. In our study, we found ZNPs in 83.3% of SRD diabetics and in 37.5% of ND subjects, with no statistically significant difference. Our results are therefore consistent with those of Goudot [4]. in the study by Nesper et al. ZNPs were found in both the superficial plexus and the choriocapillaris [9]. In contrast, Goudot et al. found no significant difference in the presence of ZNPs between SRD diabetics and controls [4]. Indeed, OCTA is unable to detect low flows of less than 0.3 m/s, so some of the ZNPs detected could correspond to areas of low blood flow and not to its complete absence.
With regard to vascular tortuosity, several studies have shown that hyperglycemia is associated with vascular dilatation [17,18]. In our study, although our patients were not hypertensive, venous tortuosity was found in 33.3% of SRD diabetics and 18.75% of ND diabetics, with no statistically significant difference between the two groups, which is in line with the series by Goudot, De Carlo and Lee [3,4,19].
In the same study, venous tortuosity was not associated with DR or DR severity [20]. Goudot, De Carlo and Lee concluded that vascular tortuosity represents a variety of the normal and cannot be considered an early detection parameter for DR [3,4,19].
Nevertheless, these studies did not specify the presence or absence of hypertension in their series, which was not the case in Vujosevic's study, who, after adjusting Blood Pressure (BP) in both groups, concluded that vascular tortuosity was more frequent in the diabetic SRD group [6].
Conclusion
OCTA was able to detect microvascular anomalies in subclinical DR, notably qualitative changes in superficial retinal microcirculation.
References
- Jalli PY, Hellstedt TJ, Immonen IJ. Early versus late staining of microaneurysms in fluorescein angiography. Retina Phila Pa. 1997; 17: 211-5.
- Cao D, Yang D, Huang Z, Zeng Y, Wang J, Hu Y, et al. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol. 2018; 55: 469-77.
- de Carlo TE. Detection of Microvascular Changes in Eyes of Patients with Diabetes but Not Clinical Diabetic Retinopathy Using Optical Coherence Tomography Angiography: Retina. 2015; 35: 2364-70.
- Goudot MM, Sikorav A, Semoun O, Miere A, Jung C, Courbebaisse B, et al. Parafoveal OCT Angiography Features in Diabetic Patients without Clinical Diabetic Retinopathy: A Qualitative and Quantitative Analysis. J Ophthalmol. 2017; 2017: 8676091.
- Tam J, Dhamdhere KP, Tiruveedhula P, Manzanera S, Barez S, Bearse MA, et al. Disruption of the Retinal Parafoveal Capillary Network in Type 2 Diabetes before the Onset of Diabetic Retinopathy. Investig Opthalmology Vis Sci. 2011; 52: 9257-9266.
- Vujosevic S, Muraca A, Alkabes M, Villani E, Cavarzeran F, Rossetti L, et al. Early Microvascular and Neural Changes In Patients With Type 1 And Type 2 Diabetes Mellitus Without Clinical Signs Of Diabetic Retinopathy. Retina Phila Pa. 2019; 39: 435-45.
- Choi W, Waheed NK, Moult EM, Adhi M, Lee B, De Carlo T, et al. Ultrahigh Speed Swept Source Optical Coherence Tomography Angiography of Retinal And Choriocapillaris Alterations in Diabetic Patients with and Without Retinopathy. Retina Phila Pa. 2017; 37: 11-21.
- Mastropasqua R, Toto L, Mastropasqua A, Aloia R, Nicola CD, Mattei PA, et al. Foveal avascular zone area and parafoveal vessel density measurements in different stages of diabetic retinopathy by optical coherence tomography angiography. Int J Ophthalmol. 2017; 10: 1545-1551.
- Nesper PL, Roberts PK, Onishi AC, Chai H, Liu L, Jampol LM, et al. Quantifying Microvascular Abnormalities With Increasing Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2017; 58: BIO307-15.
- Carnevali A, Sacconi R, Corbelli E, Tomasso L, Querques L, Zerbini G, et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017; 54: 695-702.
- Simonett JM, Scarinci F, Picconi F, Giorno P, De Geronimo D, Di Renzo A, et al. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol (Copenh). 2017; 95: e751-5.
- Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K. Quantitative Retinal Optical Coherence Tomography Angiography in Patients with Diabetes Without Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2017; 58: 190-6.
- Li Z, Alzogool M, Xiao J, Zhang S, Zeng P, Lan Y. Optical coherence tomography angiography findings of neurovascular changes in type 2 diabetes mellitus patients without clinical diabetic retinopathy. Acta Diabetol. 2018; 55: 1075-82.
- Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of Foveal Avascular Zone in Diabetic Eyes Evaluated by En Face Optical Coherence Tomography Angiography. Retina Phila Pa. 2015; 35: 2377-83.
- Hafner J, Ginner L, Karst S, Leitgeb R, Unterluggauer M, Sacu S, et al. Regional Patterns of Retinal Oxygen Saturation and Microvascular Hemodynamic Parameters Preceding Retinopathy in Patients with Type II Diabetes. Invest Ophthalmol Vis Sci. 2017; 58: 5541-7.
- Quantifying Microvascular Changes Using OCT Angiography in Diabetic Eyes without Clinical Evidence of Retinopathy. Ophthalmol Retina. 2018; 2: 418-427.
- Grunwald JE, Riva CE, Martin DB, Quint AR, Epstein PA. Effect of an insulin-induced decrease in blood glucose on the human diabetic retinal circulation. Ophthalmology. 1987; 94: 1614-20.
- Luksch A, Polak K, Matulla B, Dallinger S, Kapiotis S, Rainer G, et al. Glucose and insulin exert additive ocular and renal vasodilator effects on healthy humans. Diabetologia. 2001; 44: 95-103.
- Lee MW, Nam KY, Lim HB, Koo HM, Shin YI, Kim JY. Long-term repeatability of optical coherence tomography angiography parameters in healthy eyes. Acta Ophthalmol (Copenh). 2020; 98: e36-42.
- Sasongko MB, Wong TY, Nguyen TT, Cheung CY, Shaw JE, Wang JJ. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia. 2011; 54: 2409-16.
