
Research Article
Austin Ophthalmol. 2024; 8(3): 1069.
Real-World Efficacy of Faricimab in Treatment Resistant Neovascular Age-Related Macular Degeneration: A 12 Month Cohort Study
Evans W1,2*; Mathew C1; Eissa M1; Evans M2; Arora R1
¹Department of Ophthalmology, Salisbury District Hospital, UK
²Chester Medical School, University of Chester, UK
*Corresponding author: Evans W, Department of Ophthalmology, Salisbury District Hospital, Odstock Road, Salisbury, UK. Email: william.evans9@nhs.net
Received: December 03, 2024; Accepted: December 24, 2024; Published: December 31, 2024
Abstract
Background: Faricimab, the first bispecific anti-VEGF agent, has shown efficacy in treating neovascular Age-Related Macular Degeneration (AMD). However, existing studies often exclude anti-VEGF resistant patients, involve small sample sizes, or focus on short-term outcomes. This study aims to evaluate the real-world outcomes of faricimab in treatment-resistant neovascular AMD patients over a 12-month period.
Methods: This is a single-centre, retrospective cohort study conducted using clinical and imaging data from Salisbury district hospital, UK, between October 2022 to November 2023.
Results: A total of 191 eyes from 156 patients, with a mean age of 81.4 years and a history of 19.9 previous anti-VEGF injections per eye, were analysed. The baseline mean Best-Corrected Visual Acuity (BCVA) was 0.40 ± 0.26, and the mean Central Subfield Thickness (CST) was 283.5 μm ± 67.3 μm. After a mean follow-up of 8 months, patients received a mean of 6.49 ± 1.99 faricimab injections. A significant reduction in CST was observed, with a decrease of 283.5 ± 67.3 μm at baseline to 268.86 ± 75.06 μm at the 12-month interval, indicating anatomical improvement. Notably, the majority of patients were successfully extended to an 8-week injection interval, with a mean interval of 7.8 weeks.
Conclusions: Switching to faricimab in treatment-resistant neovascular AMD patients resulted in anatomical improvement in CST and maintenance of BCVA. These findings suggest that faricimab is a safe and effective treatment, with the potential for prolonged injection intervals. Longer-term follow-up is needed to assess the durability of these results.
Keywords: Faricimab; Neovascular age-related macular degeneration; anti-VEGF
Introduction
Age-Related Macular Degeneration (AMD) is a neurodegenerative disease estimated to affect 200 million people, making it one of the leading causes of incurable blindness worldwide [1,2]. Neovascular AMD, also known as “wet” AMD, is a late subtype characterised by the presence of choroidal neovascularization and its subsequent features, including intra- and subretinal fluid exudation and macula haemorrhages [3]. If left untreated, this condition can lead to potentially irreversible damage to photoreceptors and retinal pigment epithelium cells [4]. The primary treatment for neovascular AMD involves the suppression of Vascular Endothelial Growth Factor (VEGF), which promotes angiogenesis and vascular hyperpermeability. VEGF has been implicated in the development of neovascular AMD, and thus the development of intravitreal anti-VEGF treatments has significantly improved the management of this condition [5]. Current anti-VEGF agents approved for clinical use include ranibizumab (Lucentis), aflibercept (Eylea) and pegaptanib (Macugen) [5,6]. These treatments have already reduced the risk of legal blindless due to neovascular AMD by approximately 50% [7]. While anti-VEGF agents have revolutionized the management of wet AMD and reduced the morbidity that was previously associated with it, the incidence of wet AMD is projected to rise and the increasing burden of the disease in the population has not been met by a proportional rise in the financial or human resources required to manage the increased demand. As a result, the existing strain on AMD services nationally is expected to rise. Among the various tools at our disposal are newer anti-VEGF agents that could perhaps produce a more lasting effect and therefore reduce the treatment burden among patients thereby facilitating our ability to manage increasing demand while ensuring optimal patient outcomes.Faricimab (Vabysmo, Roche/Genentech, Basel, Switzerland) is a newly approved anti-VEGF agent by the Food and Drug Administration (FDA) as of January 2022, for the treatment of neovascular AMD [8]. Unlike previous anti-VEGF agents, faricimab is the first bispecific antibody that inhibits both VEGF-A and angiopoietin-2 (Ang-2). Ang-2, a growth factor produced in response to hypoxic stress, destabilises epithelial cells by inhibiting Ang-1 binding through the angiopoietin/tyrosine kinase pathway, leading to inflammation, vascular leakage and neovascularisation [5,7,9].
The efficacy of faricimab was demonstrated in phase 3 clinical trials (TENAYA and LUCERNE), where it was proven to be non-inferior to aflibercept in terms of best corrected visual acuity while enabling patients to be on longer treatment intervals. Indeed, approximately 80% of patients reached treatment intervals of 12 weeks or more. Additionally, improvements in anatomical outcomes were observed on Optical Coherence Tomography (OCT) compared to initial scans. Adverse effects were similar for both faricimab and aflibercept, consistent with typical expectations for this treatment [10,11]. Furthermore, phase 2 clinical studies suggested that faricimab’s dual action mechanism offers a more lasting effect compared to previous monospecific anti-VEGF agents [12].
However, clinical trials do not fully reflect the realities of realworld practice. Furthermore, both TENAYA and LUCERNE focused primarily on treatment naïve patients. In typical clinical settings, patients are often already undergoing anti-VEGF treatments. Moreover, among the larger cohort of AMD patients, there remains a sizeable contingent that have a suboptimal response to existing anti-VEGF agents and therefore, require more frequent injections. Indeed, the introduction of faricimab which purports to produce a more lasting effect compared to other anti-VEGF agents offer a promising alternative that has not been studied among treatment resistant wet AMD patients in clinical trials. Although some early real-world studies such as the multicentre TRUCKEE study include treatment resistant patients in their analysis, they are limited in that they have only assessed short term outcomes of 6 months [13- 18,20]. To our knowledge, there are very few studies examining the real-world outcomes of faricimab over an extended period among treatment-resistant patients and among the studies that do exist, these are limited to small sample sizes [19,21]. This study aims to contribute to the literature by evaluating the real-world outcomes of treatment resistant wet AMD patients switching to faricimab in a larger sample size over a 12-month period.
Methods
Inclusion and Exclusion Criteria
This retrospective observational study was performed at Salisbury District Hospital, a small district general hospital in the United Kingdom. We reviewed the data from patients who received faricimab injections between 1st October 2022 and 31st November 2023. Since the data was anonymised and collected retrospectively, informed consent was waived.
The inclusion criteria were as follows: presence of neovascular AMD in the study eye, suboptimal response to other anti-VEGF agents defined as the presence of subretinal or intraretinal fluids at 8 weeks or less, have received at least one injection of faricimab during the specified period, and have not received any other anti- VEGF agents since beginning faricimab treatment. Exclusion criteria included patients who opted out of receiving faricimab, the presence of additional retinal conditions other than AMD and significant opacities in the optical images that could compromise the quality of OCT scans. Examinations and treatments were conducted within real-world routine clinical care, following local guidelines.
Procedure and Treatment Protocol
During the specified period, patients received intravitreal faricimab injections administered by appropriately trained injectors. The procedures followed local standard protocols, involving the application of 2–3 drops of topical local anaesthesia, the use of an eye speculum, and the utilization of a 30G needle for injection at a site of 3.5mm posterior from the limbus, marked by callipers in either the supertemporal or the inferotemporal quadrant. Post-procedure antibiotics were not administered.
In our cohort, patients received faricimab according to a single defined protocol that reflected trial protocol. Patients followed a standardised regimen, receiving four monthly loading doses of faricimab, followed by a dose at 8 weeks. Subsequently, they were placed on a treat-and-extend regimen, where the injection interval was extended by 2 or 4 weeks if visual acuity was stable and notable improvements were observed in OCT scans. If there were no improvements or deterioration on visual acuity and OCT scans, the injection interval was maintained at 8 weeks or reduced by 2 or 4 weeks.
Data Collection
Clinical data was collected using the electronic patient record system Medisight (Medisoft Limited, Leeds, UK). Data extracted included patient demographics (age and sex), the affected eye, the number and type of previous anti-VEGF treatments and injections, Best Corrected Visual Acuity (BCVA), Central Retinal Subfield Thickness (CST) on Cirrus OCT, the total number of faricimab injections, and the planned treatment interval at the end of the study period.
Outcomes
The study sought to evaluate both the functional and structural outcomes of faricimab in real world clinical practice. The primary outcome was the change in Best Corrected Visual Acuity (BCVA) measured by the mean change in BCVA over time. Secondary outcomes included the mean change in central subfield thickness observed on OCT scans, the interval of injection at the end of the study period and the incidence of adverse events. Data were analysed with GraphPad Prism 9 (GraphPad Software, San Diego, CA). A Wilcoxon Signed-Rank test was used for comparisons between baseline and six months. Mean (±standard deviation) values are presented and a p-value of <0.05 was considered statistically significant.
Patient Demographics and Treatment Profile Prior to Switching
A total of 191 eyes from 156 patients met the inclusion and exclusion criteria for this study. Table 1 provides a summary of the baseline demographics and averages for these patients.
Parameters
Value
Total number of patients
156
Total number of eyes
191
Right eyes: 98 (51.3%)
Left eyes: 93 (48.7%)Gender
Female: 91 (58.3%)
Male: 65 (41.6%)Mean age (years)
81.4
Mean number of previous anti-VEGF injections in each eye
19.9
Mean number of different types of previous anti-VEGF injections in each eye
1.20
1 anti-VEGF type: 148 (77.5%)
2 anti-VEGF types: 33 (17.3%)
3 anti-VEGF types: 10 (5.2%)Types of previous anti-VEGF injections in each eye
Aflibercept: 180 (94.2%)
Ranibizumab: 41 (21.5%)
Bevacizumab: 12 (6.3%)Baseline visual acuity (VA; logMAR)
0.40
Baseline central retinal subfield thickness (CST; Ám)
283.5
Table 1: Summary of Baseline Profile of Treatment Resistant wet AMD patients prior to switch.
The average age of the study population was 81.4 ± 7.99 years, with a range from 57 to 95 years. 41.6% of patients were male (65 patients), whereas 58.3% were female (91 patients). On average, the patients had received 19.9 ± 14.57 injections in each eye before to switching to faricimab. Of the patients, 148 (77.5%) had previously received only one anti-VEGF agent, 33 (17.5%) had received two, and 10 (5.2%) had received three. The vast majority had been treated with aflibercept (94.2%) while a smaller minority had been treated with ranibizumab (21.5%) and bevacizumab (6.3%). Prior to switching to faricimab, the majority of patients were being treated with Aflibercept. The mean baseline BCVA, measured at the time of the first faricimab injection, was 0.40 ± 0.26, and the baseline mean CST was 283.5 μm ± 67.3 μm.
Following the start of loading dose of faricimab, the average period between the 1st and 2nd injections was 5.0 ± 2.5 weeks, between the 2nd and 3rd was 5.3 ± 1.9 weeks, and between the 3rd and 4th was 5.1 ± 1.5 weeks. Patients were followed up for a mean of 8 months ± 3.13 months and received a mean of 6.49 injections ± 1.99 injections. At the time of the last faricimab injection within the study period, the mean interval was 7.8 weeks. Of the 191 eyes at this stage, 34.3% were on an interval of less than 8 weeks, 54.97% were on an interval of 8 weeks to less than 12 weeks, and 10.99% were on an interval of 12 weeks or more.
Functional and Structural Outcomes
The functional outcome, vis a vis the change in best corrected visual acuity among the treatment resistant cohort is highlighted in Graph 2. The average baseline BCVA of patients before starting faricimab injections was 0.4 ± 0.26 logMAR, with a mean BCVA after the loading dose of 0.42 ± 0.26 logMAR and a mean BCVA at one year of 0.39 logMAR. No statistically significant difference in visual acuity was seen at the end of loading dose (p = 0.177).
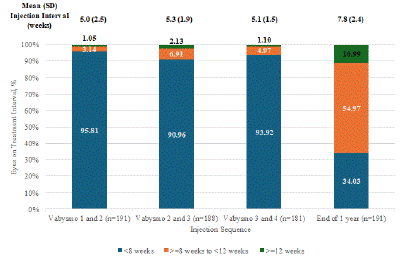
Graph 1: Mean treatment intervals among treatment resistant wet AMD patients with faricimab.
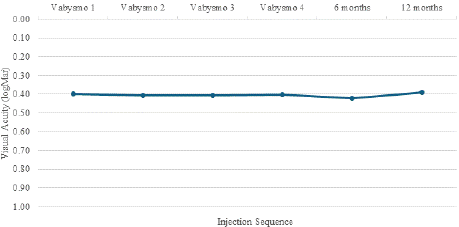
Graph 2: Change in BCVA among treatment resistant wet AMD patients
with faricimab.
The structural outcomes highlighted by change in central subfield thickness among the treatment resistant cohort is highlighted in Graph 3. There was a dramatic reduction in central subfield thickness from 283.5 ± 67.3 μm at baseline, to 242.08 ± 60.95 μm at the end of the loading phase. While some of these gains regressed at 6- and 12-month intervals, to 267.11 ± 69.8 μm and 268.86 ± 75.06 μm respectively, it never increased to the levels at baseline. There was a statistically significant reduction in CST at the end of the loading phase.
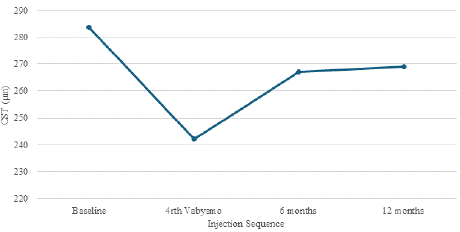
Graph 3: Change in CST among treatment resistant wet AMD patients with faricimab.
Discussion
Treatment resistant wet AMD patients are often overlooked in treatment trials and analysis of real- world data provide an opportunity to assess the impact of faricimab in this cohort. This study assessed both functional and anatomical outcomes among the treatment resistant cohort while assessing the intervals at which they were maintained at the end of the study period.
Anatomically, our study demonstrated a statistically significant improvement in central subfield thickness at the end of loading phase, at 6 months and at 1 year. This anatomical improvement aligns with findings from other studies, which reported a reduction in CST with faricimab among treatment resistant wet AMD patients, regardless of changes in BCVA [10-21]. Moreover, this mirrors the findings of both TENAYA and LUCERNE which demonstrated an improvement in central subfield thickness with Vabysmo compared to Aflibercept. Several papers have postulated that this improvement in anatomical outcomes could be attributed to the phenomenon of tolerance and tachyphylaxis among chronically active wet AMD patients who require frequent Ant-VEGF injections to maintain stability. Changes in the retinal environment in eyes with active nAMD such as increased inflammation, higher VEGF concentrations, and the development of antibodies against anti-VEGF biologics can result in an alteration of the pharmacodynamics and pharmacokinetics of the oft-used Anti- VEGF agents thereby decreasing the effectiveness of these agents over time. This process, known as tolerance, can often be overcome by increasing the dose of the drug, decreasing the intervals of anti-VEGF agents or by using drugs that target multiple signalling pathways [22- 24].
Another factor contributing to poor response to anti-VEGF agents is the pharmacological process known as tachyphylaxis, where there is a rapid decrease in drug efficacy after frequent administration due to receptor downregulation. Studies have shown that tachyphylaxis is a consequence of repetitive anti-VEGF therapy, leading to suboptimal morphological responses [22-25]. Faricimab overcomes both tolerance and tachyphylaxis by not only targeting dual signalling pathways namely the VEGF-A pathway and the Ang-2 pathway but also using a larger dose (8 mg) compared to other Anti-VEGF agents that offer a much smaller dose [19].
While anatomic outcomes demonstrated a statistically significant improvement, our study demonstrated a maintenance of best corrected visual acuity at the end of loading phase, at 6 months and at 1 year among treatment resistant wet AMD patients. This finding aligns with other real-world studies, which also reported stable maintenance of BCVA without functional improvement [12,14-16,19,20]. The lack of improvement in functional outcome may be a result of disease chronicity resulting in structural changes to the macula that limits the extent of functional improvement among treatment resistant patients. Given that the cohort in this study received more than 19 injections of Anti-VEGF prior to switching to faricimab, these patients may have had photoreceptor degeneration at baseline which limited the potential for improvement in functional outcomes.
Perhaps the most important element explored in this study was the interval at which patients were maintained at the end of the study period. Our study indicated that more than 65% of the treatment resistant cohort were extended to an interval of 8 weeks or more. The last average dosing interval for this cohort was 7.8 weeks. This is particularly significant considering that all patients in the treatment resistant cohort were previously maintained at intervals of less than 8 weeks on other Anti-VEGF agents. Our findings closely align with other studies that have demonstrated that among treatment resistant wet AMD patients, a small cohort can be extended to longer intervals [18-19,26-30].
Given the burden of frequent injections on treatment resistant patients and their caregivers, the trend towards longer intervals with faricimab is encouraging. Moreover, given that increasing demands on the AMD service nationally usually entails delayed appointment or missed appointment with a resulting negative impact on both functional and anatomic outcomes, these findings are particularly relevant. While this is promising, it must be acknowledged that there remains a sizeable cohort that remained at intervals below 8 weeks. There currently exists an unmet need for effective treatments for this cohort of treatment resistant patients, which is thought to occur due to several reasons including sub-optimal treatment intervals and due to varying drug effectiveness between different subtypes of wet AMD [22-24].
Finally, our study demonstrated no adverse effects with Vabysmo which is largely reflective of similar studies which have reported no significant differences in side effects between faricimab and other anti-VEGF agents [10-15,17-20]. The strengths of this study are that it is an analysis of the real-world outcomes among treatment resistant wet AMD patients, a cohort that is frequently overlooked in large scale trials. Moreover, the study included a large cohort and examined data over 12 months which is fairly robust for a drug that has only recently been rolled out for use in clinics nationally. To our knowledge, only two other studies have examined the outcomes of both groups over a 12-month period [19,21]. With a uniquely larger sample sizes, our study provides more accurate and representative results. But our study also has several limitations that should be acknowledged. Firstly, as a retrospective analysis, there is an inherent risk of potential biases within the data. Moreover, although the study spanned 12 months beginning October 2022, many patients did not initiate treatment at the start of the study period and the actual mean follow-up was only 8 months. Consequently, this shorter follow-up period may not be sufficient to provide robust data on the long-term outcomes of faricimab. Furthermore, as the study only evaluated the response among treatment resistant cohort, an analysis comparing the outcomes between treatment naïve and treatment resistant cohorts could not be done at this time.
Conclusions
Our study contributes to the growing literature on faricimab in real-world settings, encompassing both treatment-naïve and treatment-resistant groups. Our findings reinforce the conclusions of these previous studies and support the results that faricimab presents a viable option for treating persistent Neovascular Age-Related Macular Degeneration (nAMD) when other anti-VEGF therapies have proven insufficient. Our study indicates that faricimab is effective at maintaining best corrected visual acuity with an improvement in anatomical parameters. What’s more, a majority of the cohort were successfully extended to intervals of 8 weeks or more. Perhaps this is reflective of the ability of faricimab to overcome both tolerance and tachyphylaxis by virtue of the higher dose it entails as well as its fundamental ability to target dual signalling pathways. This suggests that other agents could also be more effective and durable at higher doses, a hypothesis supported by initial studies of Eylea 8 mg [31]. Extending treatment intervals remains a key to reducing treatment burden for patients, their caregivers as well as healthcare systems but further longer term follow ups may be necessary to evaluate if these are sustained in the longer term.
References
- Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health. 2014; 2: e106–116.
- Deng Y, Qiao L, Du M, Qu C, Wan L, Li J, et al. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes & Diseases. 2021; 9: 62-79.
- Di Carlo E, Augustin AJ. Prevention of the Onset of Age-Related Macular Degeneration. Journal of Clinical Medicine. 2021; 10: 3297.
- Ambati J, Fowler BJ. Mechanisms of Age-Related Macular Degeneration. Neuron. 2012; 75: 26–39.
- Tan CS, Ngo WK, Chay IW, Ting DS, Sadda SR. Neovascular Age-Related Macular Degeneration (nAMD): A Review of Emerging Treatment Options. Clinical Ophthalmology. 2022; 16: 917–933.
- Cheung GCM, Lai TYY, Gomi F, Ruamviboonsuk P, Koh A, Lee WK. Anti- VEGF Therapy for Neovascular AMD and Polypoidal Choroidal Vasculopathy. Asia-Pacific Journal of Ophthalmology. 2017; 6: 527-534.
- Adamis AP, Brittain CJ, Dandekar A, Hopkins JJ. Building on the success of anti-vascular endothelial growth factor therapy: a vision for the next decade. Eye. 2020; 34: 1966–1972.
- Shirley M. Faricimab: First Approval. Drugs. 2022; 82: 825-830.
- Regula JT, von Leithner PL, Foxton R, Barathi VA, Cheung CMG, Bo Tun SB, et al. Targeting key angiogenic pathways with a bispecific Cross MA b optimized for neovascular eye diseases. EMBO Molecular Medicine. 2016; 8: 1265–1288.
- Khanani AM, Heier J, Ruiz CQ, Lin H, Silverman D, Brittain C, et al. Faricimab in Neovascular Age-Related Macular Degeneration: 1-Year Efficacy, Safety, and Durability in the Phase 3 TENAYA and LUCERNE Trials. Investigative Ophthalmology & Visual Science. 2021; 62: 428.
- Heier JS, Khanani AM, Ruiz CQ, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. The Lancet. 2022; 399: 729-740.
- Khanani AM, Patel SS, Ferrone PJ, Osborne A, Sahni J, Grzeschik S, et al. Efficacy of Every Four Monthly and Quarterly Dosing of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration. JAMA Ophthalmology. 2020; 138: 964.
- Stanga PE, Valentín-Bravo FJ, Stanga SEF, Reinstein UI, Pastor-Idoate S, Downes SM. Faricimab in neovascular AMD: first report of real-world outcomes in an independent retina clinic. Eye. 2023; 37: 3282-3289.
- Kishi M, Miki A, Kamimura A, Okuda M, Matsumiya W, Imai H, et al. Short- Term Outcomes of Faricimab Treatment in Aflibercept-Refractory Eyes with Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine. 2023; 12: 5145.
- Inoda S, Takahashi H, Takahashi R, Hashimoto Y, Yoshida H, Takahashi H, et al. Visual and Anatomical Outcomes After Initial Intravitreal Faricimab Injection for Neovascular Age-Related Macular Degeneration in Patients with Prior Treatment History. Ophthalmology and Therapy. 2023; 12: 2703–2712.
- Hikichi T. Investigation of satisfaction with short-term outcomes after switching to faricimab to treat neovascular age-related macular degeneration. Japanese Journal of Ophthalmology. 2023; 67: 652–656.
- Khanani AM, Aziz AA, Khan H, Gupta A, Mojumder O, Saulebayeva A, et al. The real-world efficacy and safety of faricimab in neovascular age-related macular degeneration: the TRUCKEE study – 6 month results. Eye. 2023; 37: 3574-3581.
- Leung EH, Oh DJ, Alderson SE, Bracy J, McLeod M, Pérez LI, et al. Initial Real-World Experience with Faricimab in Treatment-Resistant Neovascular Age-Related Macular Degeneration. Clinical Ophthalmology. 2023; 17: 1287– 1293.
- Ng B, Kolli H, Kumar NA, Azzopardi M, Logeswaran A, Buensalido J, et al. Real-World Data on Faricimab Switching in Treatment-Refractory Neovascular Age-Related Macular Degeneration. Life. 2024; 14: 193.
- Schneider M, Bjerager J, Hodzic-Hadzibegovic D, Klefter ON, Subhi Y, Hajari J. Short-term outcomes of treatment switch to faricimab in patients with aflibercept-resistant neovascular age-related macular degeneration. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2024; 262: 2153-2162.
- Rush RB. One-Year Outcomes of Faricimab Treatment for Aflibercept- Resistant Neovascular Age-Related Macular Degeneration. Clinical ophthalmology. 2023; 17: 2201–2208.
- Mettu PS, Allingham MJ, Cousins SW. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Progress in Retinal and Eye Research. 2021; 82: 100906.
- Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, Gibson J, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye. 2015; 29: 721–731.
- Sun X, Yang S, Zhao J. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Design, Development and Therapy. 2016; 1857.
- Keane PA, Liakopoulos S, Ongchin SC, Heussen FM, Msutta S, Chang KT, et al. Quantitative Subanalysis of Optical Coherence Tomography after Treatment with Ranibizumab for Neovascular Age-Related Macular Degeneration. Investigative Opthalmology & Visual Science. 2008; 49: 3115.
- Rush RB, Rush SW. Intravitreal Faricimab for Aflibercept-Resistant Neovascular Age-Related Macular Degeneration. Clinical Ophthalmology. 2022; 16: 4041–4046.
- Kataoka K, Itagaki K, Hashiya N, Wakugawa S, Tanaka K, Nakayama M, et al. Six-month outcomes of switching from aflibercept to faricimab in refractory cases of neovascular age-related macular degeneration. Graefe s Archive for Clinical and Experimental Ophthalmology. 2023; 262: 43–51.
- Szigiato A, Mohan N, Talcott KE, Mammo DA, Babiuch AS, Kaiser PK, et al. Short-Term Outcomes of Faricimab in Patients with Neovascular Age-Related Macular Degeneration on Prior Anti-VEGF Therapy. Ophthalmology Retina. 2024; 8: 10–17.
- Pandit SA, Momenaei B, Wakabayashi T, Mansour HA, Vemula S, Durrani AF, et al. Clinical Outcomes of Faricimab in Patients with Previously Treated Neovascular Age-Related Macular Degeneration. Ophthalmology Retina. 2023; 8: 360-366.
- Goodchild C, Bailey C, Hernaez JS, Ahmed E, Salvatore S. Real world efficacy and durability of faricimab in patients with neovascular AMD (nAMD) who had sub-optimal response to prior anti-VEGF therapy. Eye. 2024; 38: 3059-3064.
- Lanzetta P, Korobelnik J-F, Heier JS, Leal S, Holz FG, Clark WL, et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, noninferiority, phase 3 trial. The Lancet. 2024; 403: 1141-1152.