Research Article
Austin Orthop. 2016; 1(1): 1002.
Surgery Improves Pain and Quality of Life in Multiple Myeloma Patients with Symptomatic Osteolytic Spinal Lesions
Syrimpeis VN¹*, Korovessis P¹, ZikosP² and Peter Fennema³
¹Orthopaedics Department, General Hospital of Patras, Greece
²Hematology Departments, General Hospital of Patras, Greece
³AMR Advanced Medical Research GmbH, Switzerland
*Corresponding author: Syrimpeis VN, Orthopaedics Department, General Hospital of Patras, Greece
Received: July 19, 2015; Accepted: August 29, 2016; Published: September 01, 2016
Abstract
Study Design: A prospective study.
Objective: To present the functional outcome and the survival of 21 consecutive selected Multiple Myeloma (MM) patients who underwent 25 surgeries for symptomatic vertebral body osteolysis.
Summary of Background Data: Percutaneous augmentation with polymethyl methacrylate in patients with osteoporotic vertebral body fractures safely reduces the vertebral body deformity and pain. There are few shortterm studies reporting functional outcome and survival, following surgery for osteolytic vertebral body lesions in MM patients, with or without neurological impairment.
Methods: Between December 2004 and May 2012, 25 wide spectrum surgeries including percutaneous augmentation, hybrid fixation and circumferential decompression were performed for symptomatic vertebral body osteolysis in 21 selected patients with MM. Tomita osteolysis classification, Karnofsky disability scale; ASIA neurological impairment scale and VAS pain scale were used. Survival analysis was performed.
Results: All patients were followed for a minimum of 6 months postoperatively. Karnofsky Index improved from 66%±20% preoperatively to 81.3%±15%, one month and 83%±10% one year postoperatively. VAS score significantly reduced in all patients from 7.08±2 preoperatively to 3.35±1.5 at the latest evaluation. One patient with ASIA grades D and 2 with ASIA grades C improved postoperatively to ASIA E. The one-year survival from index diagnosis was 85.2% (95% CI, 60.6% - 96.0%), while it dropped to 55.4% (29.4% - 75.1%) five-year postoperatively. Τhe one-year survival rate from index surgery was 65.9% (95% CI, 38.8% - 83.2%), and dropped to 33.5% (95% CI, 11.1% - 58.0%) five-year post operation.
Conclusions: There are several modalities of surgery for symptomatic osteolytic vertebral body lesions in MM patients. Surgery was proved a safe procedure with few complications it reduced pain and improved quality of life. Together with hematological and radiation therapy it may increase the survival of MM patients.
Keywords: Multiple Myeloma; Spinal Lesions; Spine Surgery; Augmentation; Decompression; Spine; Minimal Invasive Surgery; Stabilization; Kyphoplasty; Tumor
Introduction
Multiple Myeloma (MM) is a systemic neoplasm of plasma cells that affects 1-4 per 100,000 people per year and is commonly associated with bone pain, usually due to spinal and rib osteolyses, in 70% of this kind of patients [1-4]. Skeletal osteolyses are the most frequent cause of morbidity and mortality in patients affected by this pathology [5].
Spinal involvement can be the initial clinical presentation of the disease in 34-64% of the MM patients, leading often to intractable pain and/or neurological complications due to spinal cord or cauda compression [6,7]. In the one third of the patients, MM is diagnosed after a pathological spinal fracture has occurred [8], moreover new vertebral body fractures occur in approximately 15-30% of patients with MM annually [5].
Recent advances in therapeutic approaches, such as autologous stem cell transplantation, radiotherapy and chemotherapy, bracing and surgery in certain cases, helps towards lessening the occurrence and severity of adverse effects of this disease, as well as managing associated complications. [7,9-14]. Although medical treatments & radiation help towards slowing down the natural history of MM [5], they do not correct any structural vertebral destruction that may have already been occurred, either as osteolysis or as a fracture and wedge deformity in up to 70% of all patients with MM [15-17]. In vertebral body osteolyses and/or vertebral body fractures, the main goal of surgical intervention is pain relief, reduction of angular deformity for prevention of potential neural element compression and spinal canal decompression. In the last few years, percutaneous Minimal Invasive Surgery (MIS), vertebral augmentation techniques such as Vertebroplasty (VP), Balloon Kyphoplasty (BK) and KIVA [18], are well tolerated and drastically decrease pain while simultaneously improve patient’s quality of life [15,16,17,19]. Radiofrequencytargeted vertebral augmentation was recently developed to address potential adverse issues reported with VP and BK [2,20,21,22]. However, in patients with vertebral body osteolyses with involvement of the posterior vertebral body wall some authors have raised concerns regarding the high leakage rates associated with low viscosity polymethylmethacrylate (PMMA) bone cement [23,24,25,26].
In cases of pathologic vertebral body fracture associated spinal canal encroachment with or potential for neurological involvement, open decompressive surgeries with stabilization may be indicated, however these are depending on the general patient’s condition which in MM patients is often poor.
Survival after MM is highly variable; however, recent studies of various drug therapies have led to promising outcomes and reported survival beyond 10 years [12-13].
Although early clinical results are promising [27], there is no evidence regarding long-term effect of palliative surgery in MM patients with symptomatic vertebral osteolysis.
The aim of this prospective study is to present the functional outcome and survival rates following surgical treatment in 21 consecutive selected MM patients, who underwent a total of 25 surgeries, by a single senior orthopedic spine surgeon, in one tertiary institution and to review the relative literature.
Materials and Methods
Twenty-one consecutive selected patients (7 women, 14 men) suffering from MM with established spinal involvement and associated intractable pain, who were surgically treated between 2004 and 2012 in the author’s Orthopaedic institution by a single spine surgeon (Table 1), were prospectively evaluated. Institutional Review Board (IRB) approval and patient informed consent was obtained in all patients. The average ±SD age of the patients at the index surgery was 70±21, range 49-90 years. All patients were managed by a multidisciplinary team including hematologist, radiotherapist and orthopaedic spine surgeon. Systemic therapy (chemotherapy & irradiation) was administrated in 19/21 patients before and/or after surgery. Bone marrow transplantation before surgery had been done in 1/21 patient. Preoperative patient evaluation included a complete physical examination, plain roentgenograms, CT-scan, Magnetic Resonance Imaging (MRI) and hematological evaluation. The Tomita classification [31] was used to grade the extension of vertebral body osteolytic lesions, (Table1). The VAS (0-10 scale) [28] and the ASIA neurological classification [29] were used for evaluation of patients’ pain level and neurological function. The quality of life was evaluated with the Karnofsky Index [30].All values are expressed as average ±SD. The inclusion criteria and indications for surgical intervention were MM or solitary spinal plasmocytoma with symptomatic spinal involvement (painful osteolysis ± spinal fracture, neurological impairment or potential or progressive neurological impairment due to vertebral body fracture), intractable spinal pain resistant to conservative treatment (pain killers, brace, etc). The diagnosis of MM was already preoperatively established in 17/25 (68%) cases, while in the remaining 8/25 (32%) cases, the diagnosis was first disclosed from the intra-operatively taken biopsy. Our surgical strategy was as follows: Patients neurologically intact and osteolysis in ≥1 non-contiguous vertebral body (-ies) were treated with vertebral augmentation solely; in patients with multilevel contiguous cervical spine involvement vertebrectomy, mesh cage plus posterior fixation was made; patients with neurologic impairment were treated with posterior MIS reduction, pedicle screw stabilization plus vertebral body augmentation; patients with posterior cord/cauda compression (posterior spinal elements involvement) were treated via wide laminectomy and posterior pedicle screw fixation. Patient survival, using all-cause mortality as event of interest, was estimated with the Kaplan-Meier method [32].
Case No
Age at Surgery
Gender
Tomita Osteolysis Grade
Neurological Impairment on Admission
Location of Osteolyses (Fractures are Indicated)
Surgical Treatment
Survival from Diagnosis (Days)
Survival from Surgery (Days)
1
65
F
Type 6
No
C3, C5, C6
Combined Staged 360o (Post. C2-C6 & Anterior Decompression C3, C5, C6 with Mesh Cage)
2386
2401
2
83
F
Type 6
Paraparesis Incomplete
L3, L4, L5
Mis Post. Stab L3-L5
559
650
3
73
Μ
Type 7
No
Fractures in T5, T8, T9, T10, T11
Augmentation: T5, T8, T9, T10, T11
2566
2570
4
73
Μ
Type 7
No
Fractures in T7, T12, L1, L2
Augmentation: T7, T12, L1, L2
432
385
5
53
M
Type 7
Paraparesis Incomplete
Fractures in T8, T10, L1, L2, L3
Hybrid Fixation Augmentation: L1, L2, L3, Decompression & Post. Stab. T7-L3
508
498
6.1
68
F
Type 1
No
T11
Augmentation: T11
2990
414
6.2
69
F
Type 6
No
T9, T10, T11
Augmentation: T9, T10
2990
217
7
73
F
Type 6
No
Fractures in L2, L3, L4
Augmentation: L2, L3, L4
3486
339
8
63
Μ
Type 6
No
L2-L5
Augmentation: L2, L3, L4, L5
1299
226
9
78
F
Type 7
Paraparesis Incomplete
Fractures in T11, L1
Hybrid Fixation Augmentation: T11, O1 Mis Post. Stab. T12-L2
38
31
10
81
Μ
Type 7
No
Fractures in T11, T12, L4, L5
Hybrid Fixation Augmentation: T11, T12, L4, L5 Decompression & Post. Stab. T10-L2
422
349
11
70
Μ
Type 6
No
L1, L2, L3, L4
Augmentation: L1, L2, L3, L4
1222
553
12.1
49
Μ
Type 4
Paraparesis Incomplete
Fracture in T6 with Epidural Metastasis
Decompression & Post. Stab. T3-T8
1043
897
12.2
51
Μ
Type 6
No
Fracture In T2
Extension of Post. Stab. To T1
1043
165
13
65
Μ
Type 7
No
Fractures in T11, T12, L2, L3, L4
Augmentation: L2, L3, T11, T12, L4
1293
617
14
83
F
Type 7
Paraparesis Incomplete
Fractures in T12, L1, L4, L5
Augmentation: T12, L1, L4, L5
3276
425
15
77
Μ
Type 3
No
Fracture in L3
Hybrid Fixation Augmentation: L3 Mis Post. Stab. L2-L4
3471
435
16
78
F
Type 6
No
Fractures in T12, L1
Augmentation: T12, L1
414
420
17
75
M
Type 7
No
Fractures in L1, L2 Osteolyses in T10, T11, T12, L3
Hybrid Fixation Augmentation: L1, L2 Post. Stab. T8-L5
968
687
18.1
64
Μ
Type 3
No
Fracture in L3
Hybrid Fixation Augmentation: L3 Mis Post. Stab. L2-L4
3236
3250
18.2
69
Μ
Type 6
Paraparesis Incomplete
Fracture in L5, Osteolyses in S1, S2
Augmentation: L5, S1, S2
3236
1295
19
78
Μ
Type 7
Paraparesis Incomplete
Fractures in L2, L3, T7, T8, with Epidural Extension
Hybrid Fixation Augmentation: L2, L3, T7, T8 Mis Post. Stab. T12-L4
14
21
20
90
Μ
Type 6
No
Fractures in L2, L3
Augmentation: L2, L3
249
286
21.1
63
Μ
Type 7
No
T2, T7, T8
Augmentation: T7
1732
1760
21.2
64
Μ
Type 7
Paraparesis Incomplete
Fractures in C7, T2, T3, T6, T7, T10, T11, L1
Post. Stab. C5-T4
1732
1534
Table 1: Cumulative data on 21 MM patients who underwent 25 surgeries for painful vertebral osteolyses. Four patients underwent two subsequent surgeries for other level osteolyses. Patients no 6, 12, 18 & 21 were operated twice. (F=female & M=male).
Survivals from: a) Index MM diagnosis and b) Index surgery were calculated. All survived patients underwent a personal interview by an independent orthopaedic surgeon, who did not participate in the operations and included physical examination plus imaging study 3, 6 and 12 months post-operatively and thereafter once annually.
Results
The most common spinal location of vertebral body osteolysis was the thoracolumbar junction (16/25 cases), and the less common was the cervical spine with only 1 case (4%). Multilevel spinal localization was observed in 9/25 cases (Table 1).
Epidural MM localization (posterior elements involvement) associated with ASIA grades C was present in one patient with thoracic lesion (case 12.1, Table 1) and in another patient with lesions both in the thoracic & lumbar spine (case 19, Table 1).
Percutaneous augmentation was performed in the majority of the cases: 13/25 (52%); followed by hybrid MIS in 7/25 cases (28%); and posterior pedicle screw fixation in 4/25 cases (16%). Combined open anterior decompression corpectomy and mesh cage implantation supplemented by posterior lateral mass stabilization for multi-level cervical osteolytic lesions and associated kyphotic deformity was performed in one female patient (4%) for cervical kyphosis and potential for cervical spinal cord compression (Table 1).
There were no refractures at the levels of previously augmented vertebrae and no patient was re-operated because of recurrence of the osteolysis at the already augmented vertebral bodies.
Four from the 21 patients, were re-operated at different spinal levels for new symptomatic vertebral body osteolyses and/or associated fractures (Table 1). One patient (cases No. 6.1 & 6.2, Table 1), with previous augmentation of T11-vertebra was re-operated 6 months later because of pain in two adjacent vertebrae (T9 and T10), (Table 1). In one additional patient (Case No. 12.1 & 12.2,Table1), a cephalad extension of an already existed posterior pedicle screw construct was made for new T2 vertebral body osteolysis, 24.5 months following primary decompression and posterior stabilization for severe osteolytic lesion in a lower level (Table 1).
Five patients (Case 2, 9, 15, 18.1 & 19) were treated with MIS with or without simultaneous vertebral augmentation (Table 1).
The time lapsed from the index diagnosis to index surgery, for the 17/25 (68%) cases for which the diagnosis was already preoperatively known was 40±6.15 months (Range 0.25-105).
Functional results
Daily performance (Karnofsky Index) was significantly improved from 66%±20% before surgery to 81.3%±15% one month following surgery and 83%±10%, one year after surgery in survived patients, (Table 2).
TABLECREATED
Table 2: Karnofsky Index preoperatively, 1 month and 1 year post-operatively, ASIA Impairment Scale and VAS Axial Pain Scale pre-operatively and post-operatively & postoperative complications & complications outcome.
Two patients (Case No: 9 & 19, Table 2) were excluded from pain evaluation because they died earlier. Pain relief was achieved in patients who survived for more than one month following surgery. VAS score was reduced from 7.08±2 preoperatively to 3.35±1.5 at the time of last postoperative evaluation.
No neurological deterioration was observed postoperatively in 18/19 patients with preoperative ASIA grades E and D. One patient (Case-5, Table 2) with preoperative ASIA grade D and 2 patients, cases 12.1 & 21.2, with ASIA C grades improved postoperatively to ASIA E, Table 2.
Survivorship
The one-year survival from the index diagnosis was 85.2% (95% CI, 60.6% - 96.0%), while the 5 year survival dropped to 55.4% (95% CI, 29.4% - 75.1%), see (Figure 1).
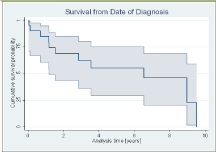
Figure 1: Survival from date of diagnosis.
The one-year survival from the index surgery, was 65.9% (95% CI, 38.8% - 83.2%), while the five year survival dropped to 33.5% (11.1% - 58.0%), see (Figure 2).
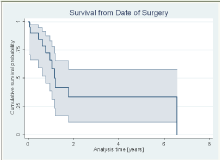
Figure 2: Survival from date of surgery.
Complications
General: No death was recorded in any patient in the early postoperative period. No lung embolism or deep vein thrombosis were shown in any patient.
In case No. 9, the patient died 31 days after surgery since the general condition of the patient due to final stage MM was significantly affected (Table 2).
There was no extraordinary perioperative bleeding related to MM.
Acute renal failure occurred in two patients: In Case-5, the acute renal failure was successfully controlled with medication, while in the case 19 the patient died 21 days after surgery from multiple organ failure (Table 2). This particular patient (Case-19) presented already preoperatively with incomplete paraplegia (ASIA C) and had several osteolyses with epidural affection in the thoraco-lumbar spine, (Table 1).
Surgical: There were no deep infections or instrumentationrelated complications.
In Case No. 15, there was developed, immediately postoperatively, an L4 weakness (2/5) due cement leakage into the foramina, in a patient with severe (Tomita 3) vertebral body bone erosion that caused temporary nerve root irritation and resolved one month later (Table 2).
Discussion
Multiple Myeloma is the most common primary hemopoietic tumor with osteolytic spinal localization and associated complaints (pain, disability, potential for neurologic impairment etc) [8].
With the advances in chemotherapy, radiotherapy and the use of autologous peripheral stem cell grafts in the last 10 years, the prognosis of ΜΜ patients has significantly improved. The reported median survival time from the index diagnosis has increased from an average of 2.5 to 4.5 years [21,33]. The one-year survival in our patients from index diagnosis was 85.2% (95% CI, 60.6% - 96.0%), while the five-year survival dropped to 55.4% (29.4% - 75.1%).
Besides medical treatment of MM, several palliative surgical methods (BK, VP, KIVA, open decompression, pedicle screw fixation, etc) have been introduced to reduce pain and improve quality of life in MM patients with and without neurological impairment. In MM patients, major surgery often cannot be safely performed because of systemic complications associated with MM (renal insufficiency, etc) therefore MIS techniques that stabilize painful spinal osteolyses are currently used with sufficiently good reported results.
Expansible vertebral body osteolyses and fractures with associated wedge deformity and spinal instability are quite often present (75%) in MM patients [34] and may result in compression of spinal cord or cauda leading to neurological impairment. In our study population, neurological impairment was present on admission in 6/21 (28.9%) MM patients, slightly higher than those previously reported (22% to 25%) [11,13]. All 6 patients with preoperative neurologic impairment improved at least one ASIA grade while no patient deteriorated postoperatively.
Only few studies are reported, mostly case series, with retrospective design report on the clinical course of small groups of surgically treated MM patients [35,36,37]. The high benefits of surgery in symptomatic MM patients with spinal involvement seems to be the lower surgical complication rate (8%) [43] Than the one observed in patients with metastatic spinal disease (19%) [44].
Our prospective study reports on a consecutive homogenous selected series of 21 patients with MM, who successfully underwent surgery for symptomatic spinal osteolyses and showed a significant decrease of pain and improvement in quality of life in all patients with few complications. A recent study [43] on the treatment of MM patients suffering from osteolytic vertebral body fractures treated with combined BK and radiofrequency showed a significant reduction of VAS score from 8.1 to 2.5, with an average reduction of preoperative VAS of 5.6 points in 75% of the operated patients. In our series, pain relief was achieved in all 23 Cases that survived for more than 30 days postoperatively. VAS was reduced from 7.08±2 preoperatively to 3.35±1.5 at the time of postoperative evaluation.
Choe et al [41] reported on a 4.6% incidence of pulmonary embolism in patients with MM after VP or BK with a high correlation between PMMA in the lungs and paravertebral PMMA leak, independent of treatment type (VP or BK). In no patient in our series lung embolism was clinically evident. However, in our series, complications of lower severity occurred in 3/25 surgeries (12%) -3/21 patients- and included acute renal insufficiency and transient lower limb muscle weakness. Our complication rate is significantly lower to those previously published of approximately 37.5% in [38].
During vertebral body augmentation, surgeons are often facing pulmonary and neurologic complications related to PMMA extravasation. In MM patients, PMMA extravasation rates following VP ranges from 1% to 48%, while it is less common in BK (<2%) [15,16,39,40]. Recently, Julka et al reported cement extravasation in 12/32 (37.5%) patients, all without clinical sequelae [38]. In 52 VPs in 37 MM patients, vertebral augmentation reported in 3/37 (8%) patients with transient nerve root paresis because of cement leakage, while 1/37 (2.7%) patient required nerve root decompression with PMMA removal [42]. In our series, there was only one case with cement leakage into the foramina, in a patient (Case 15) with severe (Tomita 3) vertebral body bone erosion that caused temporary nerve root irritation and resolved one month later.
Surgery in the cervical vertebrae affected by MM was performed only by ventral decompression and stabilization [43]. Owing to the risk of vertebral instability, decompressive laminectomy alone was not indicated [43]. In our single case with cervical spine involvement and good general health a combined 360o surgery was performed to decompress and simultaneous stabilize the cervical spine for multilevel involvement and increasing kyphotic deformity due to osteolysis. This particular patient survived for 6.5 years and died because of pharynx cancer.
The one-year survival rate from the date of surgery was 65.9% (95% CI, 38.8% - 83.2%), while the five-year survival rate dropped to 33.5% (95% CI, 11.1% - 58.0%). The most common cause of death following palliative surgery was multiple organ failure because of the MM in final stage.
Formal laminectomy alone is usually not recommended for decompression and osteolysis treatment in metastatic or MM patients, because a wide posterior decompression further destabilizes the spine. Laminectomy combined with stabilization was reserved in four patients with posterior spinal canal encroachment due to posterior elements involvement and dural compression. Consistent with previous studies [45,46,37], spinal instability due to vertebral body osteolyses, associated with intractable pain and potential for neurologic impairment were the indications for surgery in our patients. Surgery performed in our MM patients, was patientspecific and ranged from percutaneous augmentation with PMMA to MIS pedicle screw fixation combined with vertebral augmentation with PMMA, anterior open decompression and combined anterior decompression plus posterior pedicle screw fixation. In MM patients with neurologic impairment due to epidural compression by the MM lesion itself, without structural deficiency of the vertebral body, radiation is often able to diminish the local tumor lesion and the associated axial pain. However, radiation therapy alone cannot treat instability induced by vertebral body osteolysis and associated pathological fractures. Spinal instability resulted from vertebral body osteolysis requires mechanical stabilization to reduce axial pain and simultaneously to prevent potentially secondary neurological impairment due to spinal cord and cauda compression.
There are two strengths in our study. The first strength is the homogenous population with only pure MM patients. In the relative literature, most studies reported on mixed populations of MM and cancer patients [15,47]. The second strength is that all patients were operated by one senior experienced orthopaedic spine surgeon.
There are some drawbacks associated with MIS techniques used in MM patients. Radiation exposure of both patient and operation personnel can occur at various adverse levels and risk [48,39]. In order to monitor potential epidural PMMA extravasation or PMMA embolization during PMMA delivery, VP and BK procedures require significant fluoroscopy time.
References
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. “Multiplemyeloma”. Lancet. 2009; 374: 324-339.
- Mont’Alverne F, Vallée JN, Guillevin R, Cormier E, Jean B, Rose M, Caldas JG, Chiras J. “Percutaneous vertebroplasty for multiple myeloma of the cervical spine”. Neuroradiology. 2009. 51: 237-242.
- Longo DL. “Treatment of advanced Hodgkin lymphoma: The more things change, the more they stay the same”. Journal of clinical oncology : Official journal of the American Society of Clinical Oncology. 2013: 31: 660-662.
- Harrison’s Principles of Internal Medicine. 18: 938.
- Coleman RE. “Clinical features of metastatic bone disease and risk of skeletal morbidity”. Clinical cancer research : An official journal of the American Association for Cancer Research. 2006; 12: 6243-6249.
- Cortet B, Cotten A, Boutry N, Dewatre F, Flipo RM, Duquesnoy B, et al. “Percutaneous vertebroplasty in patients with osteolytic metastases or multiple myeloma”. Revue du rhumatisme (English ed.). 1997; 64: 177-183.
- Mundy GR. “Myeloma bone disease”. European journal of cancer (Oxford, England : 1990). 1998; 34: 246-251.
- Weinstein JN, McLain RF. “Primary tumors of the spine”. Spine. 1987; 12: 843-851.
- Ocio EM, Mateos MV, Maiso P, Pandiella A, San-Miguel JF. “New drugs in multiple myeloma: Mechanisms of action and phase I/II clinical findings”. The lancet oncology. 2008; 9: 1157-1165.
- Palumbo and Rajkumar SV. “Treatment of newly diagnosed myeloma”. Leukemia. 2009; 23: 449-456.
- Bensinger WI. “Role of autologous and allogeneic stem cell transplantation in myeloma”. Leukemia. 2009; 23: 442-448.
- Tamburrelli FC, Proietti L, Scaramuzzo L, De Stefano V, Logroscino CA. “Bisphosphonate therapy in multiple myeloma in preventing vertebral collapses: preliminary report”. European spine journal : Official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2012; 21: 141- 145.
- Barlogie B, Shaughnessy J, Tricot G, Jacobson J, Zangari M, Anaissie E, et al. “Treatment of multiple myeloma”. Blood. 2004; 103: 20-32.
- Yeh HS, Berenson JR. “Treatment for myeloma bone disease”. Clinical cancer research : An official journal of the American Association for Cancer Research. 2006; 12: 6279-6284.
- Dudeney S, Lieberman IH, Reinhardt MK, Hussein M. “Kyphoplasty in the treatment of osteolytic vertebral compression fractures as a result of multiple myeloma”. Journal of clinical oncology : Official journal of the American Society of Clinical Oncology. 2002; 20: 2382-2387.
- Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrar K, et al. “Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients”. Journal of neurosurgery. 2003; 98: 21-30.
- Hadjipavlou G, Tzermiadianos MN, Katonis PG, Szpalski M. “Percutaneous vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures and osteolytic tumours”. The Journal of bone and joint surgery. British volume. 2005; 87: 1595-1604.
- Korovessis P, Repantis T, Miller LE, Block JE. “Initial clinical experience with a novel vertebral augmentation system for treatment of symptomatic vertebral compression fractures: A case series of 26 consecutive patients”. BMC musculoskeletal disorders. 2011; 12: 206.
- McGirt MJ, Parker SL, Wolinsky JP, Witham TF, Bydon A, d Gokasla ZL. “Vertebroplasty and kyphoplasty for the treatment of vertebral compression fractures: An evidenced-based review of the literature”. The spine journal : Official journal of the North American Spine Society. 2009; 9: 501-508.
- Chiras J, Depriester C, Weill A, Sola-Martinez MT, Deramond H. “Percutaneous vertebral surgery. Technics and indications”. Journal of neuroradiology. Journal de neuroradiologie. 1997; 24: 45-59.
- Hrabálek L, Bacovský J, Scudla V, Wanek T, Kalita O. “Multiple spinal myeloma and its surgical management”. Rozhledy v chirurgii : měsíčník Československé chirurgické společnosti. 2011; 90: 270-276.
- La Maida GA, Giarratana LS, Acerbi A, Ferrari V, Mineo GV, Misaggi B. “Cement leakage: safety of minimally invasive surgical techniques in the treatment of multiple myeloma vertebral lesions”. European spine journal : Official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society, 2012; 21: 61-68.
- Gerszten PC, Monaco EA. “Complete percutaneous treatment of vertebral body tumors causing spinal canal compromise using a transpedicular cavitation, cement augmentation, and radiosurgical technique”. Neurosurgical focus. 2009; 27: E9.
- Gerszten PC, Welch WC. “Combined percutaneous transpedicular tumor debulking and kyphoplasty for pathological compression fractures. Technical note”. Journal of Neurosurgery. Spine. 2007; 6: 92-95.
- Halpin RJ, Bendok BR, Liu JC. “Minimally invasive treatments for spinal metastases: vertebroplasty, kyphoplasty, and radiofrequency ablation”. The journal of supportive oncology. 2: 339-351; discussion 352-355.
- La Maida GA, Giarratana LS, Acerbi A, Ferrari V, Mineo GV, Misaggi B. “Cement leakage: safety of minimally invasive surgical techniques in the treatment of multiple myeloma vertebral lesions”. European spine journal : Official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2012; 21: 61-68.
- Levine SA, Perin LA, Hayes D, Hayes WS. “An evidence-based evaluation of percutaneous vertebroplasty”. Managed care (Langhorne, Pa.). 2000; 9: 56-60,63.
- Huskisson EC. “Measurement of Pain”. The Journal of rheumatology. 1982; 9: 768-769.
- American Spinal Injury Association (ASIA).
- David AK, Walter HA, Lloyd FC. “The use of the nitrogen mustards in the palliative treatment of Carcinoma”. Cancer. 1948; 1: 634-656.
- Tomita K, Kawahara N, Baba H, Tsuchiya H, Nagata S, Toribatake Y. “Total en bloc spondylectomy for solitary spinal metastases”. International orthopaedics. 1994; 18: 291-298.
- Kaplan EL. “Nonparametric Estimation from Incomplete Observations”. Journal of the American Statistical Association. 1958; 53: 457-481.
- Von der Hoeh NH, Tschoeke SK, Gulow J, Voelker A, Siebolts U, Heyde CE. “Total spondylectomy for solitary bone plasmacytoma of the lumbar spine in a young woman: A case report and review of literature”. European spine journal : Official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2013; 23: 35-39.
- Body JJ. “Effectiveness and cost of bisphosphonate therapy in tumor bone disease”. Cancer. 2003; 97: 859-865.
- Kivioja AH, Karaharju EO, Elomaa I, Böhling TO. “Surgical treatment of myeloma of bone”. European journal of cancer (Oxford, England : 1990). 1992; 28: 1865-1869.
- Papagelopoulos PJ, Galanis EC, Greipp PR, Sim FH. “Prosthetic hip replacement for pathologic or impending pathologic fractures in myeloma”. Clinical orthopaedics and related research. 1997; 341: 192-205.
- Dürr HR, Wegener B, Krödel A, Müller PE, Jansson V, Refior HJ. “Multiple myeloma: Surgery of the spine: retrospective analysis of 27 patients”. Spine. 2002; 27: 320-324; discussion 325-326.
- Julka, Tolhurst SR, Srinivasan RC, Graziano GP. “Functional outcomes and height restoration for Patients with multiple myeloma-related osteolytic vertebral compression fractures treated with Kyphoplasty”. Journal of spinal disorders & techniques. 2014; 27: 342-346.
- Burton W, Rhines LD, Mendel E. “Vertebroplasty and kyphoplasty: A comprehensive review”. Neurosurgical focus. 2005; 18: 1.
- Barr JD, Barr MS, Lemley TJ, McCann RM. “Percutaneous vertebroplasty for pain relief and spinal stabilization”. Spine. 2000; 25: 923-928.
- Choe DH, Marom EM, Ahrar K, Truong MT, Madewell JE. “Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty”. AJR. American journal of roentgenology. 2004; 183: 1097- 1102.
- Weill, Chiras J, Simon JM, Rose M, Sola-Martinez T, E. Enkaoua, “Spinal metastases: Indications for and results of percutaneous injection of acrylic surgical cement”. Radiology. 1996; 199: 241-247.
- Zeifang F, Zahlten-Hinguranage A, Goldschmidt H, Cremer F, Bernd L, Sabo D. “Long-term survival after surgical intervention for bone disease in multiple myeloma”. Annals of oncology : Official journal of the European Society for Medical Oncology / ESMO. 2005; 16: 222-227.
- Pascal-Moussellard H, Broc G, Pointillart V, Siméon F, Vital JM, Sénégas. “Complications of vertebral metastasis surgery”. European spine journal : Official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 1998; 7: 438-444.
- Jónsson, Sjöström L, Jónsson H, Karlström G. “Surgery for multiple myeloma of the spine. A retrospective analysis of 12 patients”. Acta orthopaedica Scandinavica. 1992; 63: 192-194.
- McLain RF, Weinstein JN. “Solitary plasmacytomas of the spine: A review of 84 cases”. Journal of spinal disorders. 1989; 2: 69-74.
- Olerud and B. Jonsson, “Surgical palliation of symptomatic spinal metastases”. Acta orthopaedica Scandinavica. 1996; 67: 513-522.
- Perisinakis K, Damilakis J, Theocharopoulos N, Papadokostakis G, Hadjipavlou A, Gourtsoyiannis N. “Patient exposure and associated radiation risks from fluoroscopically guided vertebroplasty or kyphoplasty”. Radiology. 2004; 232: 701-707.