
Special Article - Adenocarcinoma
Austin Pathol. 2019; 2(1): 1005.
Mucinous Adenocarcinoma of Bladder; a Primitive not to Disregard
Bourhroum N1,3*, Lahbali O1, Ziani I2,3, Zoughari S2,3, Nouini2,3 and Znati K1,3
1Department of Pathology, Ibn Sina Hospital, University in Rabat, Morocco
2Department of Urology, Ibn Sina Hospital, University in Rabat, Morocco
3Faculty of Medicine and Pharmacy, University Mohamed V Rabat. Morocco
*Corresponding author: Bourhroum N, Department of pathology; Ibn Sina Hospital, University Mohamed V Rabat, Morocco
Received: October 10, 2018; Accepted: April 10, 2019; Published: April 17, 2019
Abstract
Adenocarcinoma is a rare tumor of bladder, which can be primitive as well as secondary. It presents similar histologic features, but it can be distinguished from metastatic adenocarcinoma by an attentive anatomo-pathological analysis with the help of results of immunohistochemistry. Primary bladder adenocarcinoma has a poor prognosis in large part because it is usually diagnosed at an advanced stage, in contrast to adenocarcinoma of urachus. Our aim, throughout this case, is to provide updated information on this entity, by putting emphasis on pathological features, differential diagnosis and clinical relevance.
Keywords: Mucinous; Bladder; Primitive
Introduction
Bladder cancer is the second most common tumor in genitourinary tract. Adenocarcinoma represent less than 2% of all bladder cancers, mucinous variant is even more rare. They are characterized by a very aggressive behaviour and are not very sensitive to radio and chemotherapy [1]. They exhibit histological features similar to those from other sites and adenocarcinoma of urachus, making diagnosis difficult, hence the primary role of immunohistochemistry in determining primitive site. Because of its rarity and these difficulties, diagnosis is always delayed.
Case Presentation
We report the case of an 80-year-old woman with no pathological history who consulted for total hematuria. The physical examination revealed a slight hypogastric sensibility.
Ultrasound showed a thickening of the bladder wall with a heterogeneous content from which the indication of a computerized tomography scan that showed presence of a bladder tumor process without repercussion on the upper urinary tract (Figure 1,2).
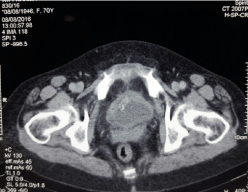
Figure 1: Tissue process of the vesical wall.
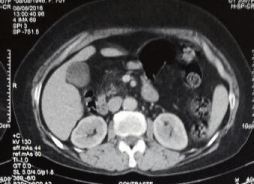
Figure 2: No impact on Kidney.
A transurethral resection of the tumor is performed. Pathological study suggested the initial diagnosis of a mucinous adenocarcinoma infiltrating bladder muscle (Figure 3-5). An immunohistochemical study was performed, having objectified the positivity of tumor cells to cytokeratin 20 but cytokeratin 7 was negative. Cytoplasmic staining of the tumor cells with loss of nuclear expression of beta Cathenin confirmed the primitive character (Figure 6-8). Patient was readmitted after 6 months for radical treatment. She had a pelvic anterior flap with an lymph node iliac dissection and a urinary diversion with cutaneous ureterostomy.
Macroscopic examination of the operative specimen showed presence of a friable budding tumor at the surface of bladder wall with a mucoid appearance at the cut. It infiltrates the bladder muscle. Vesico-uterine cul de sac is free.
Microscopic examination revealed a carcinomatous proliferation, made of glandular structures and cellular nests bathed in large puddles of mucus with presence of papillary projections surrounded by a muco-secreting columnar epithelium with moderate cytonuclear atypia. Proliferation infiltrates bladder muscle. Glandular surface metaplasia is present without carcinoma in situ. Absence of vascular embolism and perinervous encasement. Lymph node dissection was free from tumor infiltration (Figure 3-5). Histological and immunohistochemical evaluation thus confirmed diagnosis of primary mucinous adenocarcinoma of bladder without extra-vesical or lymph node propagation (Figure 6,7). The extension assessment did not objectify metastasis. A three-month control CT scan, did not show metastasis. A clinical and radiological monitoring every 6 months was adopted.
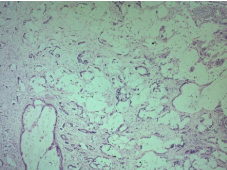
Figure 3: Extensive extracellular pools of mucin with malignant glands and
cells floating on it HEX5.
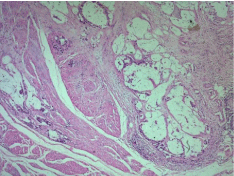
Figure 4: Malignant glandsr infiltrates vesical muscle HEX5.
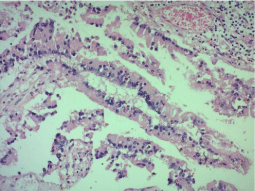
Figure 5: Papillary structures replacing the urothelium HEX20.
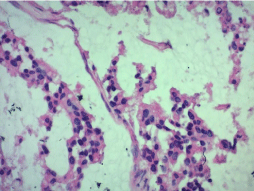
Figure 6: Tumor cells with atypical nuclei HEX40.
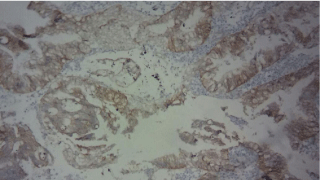
Figure 7: CK 20 positive in tumor cells X40.
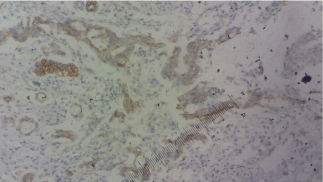
Figure 8: Membranous and weak cytoplasmic expression of Beta-catenine
X40.
Discussion
Primary adenocarcinoma of bladder exists in many forms, including enteric, signet ring cell, clear-cell and mixed-forms. Adenocarcinoma of the urachus is much more common. The mucinous variant is a rare entity, accounting for less than 2% of all bladder cancers [1]. On the other hand, no prognostic impact on the survival of this classification of bladder adenocarcinoma has been shown, even though the survival curve of adenocarcinoma in “signet ring cell” has a very steep downward slope during the first two years [2].
It sits most often in the dome of the bladder, the trine and the side wall. There are three hypotheses concerning the histological origin: Many authors suggest that adenocarcinoma occur following intestinal metaplasia stimulated by chronic irritation, this hypothesis can be an explanation for the high incidence of adenocarcinoma in populations exposed to schistosomiasis. Others suggest that the glands of the embryonic remnants remain in the transition epithelium at the level of the trigone, but would not explain the other locations [3]. Finally, the discovery of transitional cells associated with adenocarcinoma supports the hypothesis that this tumor, like the others, develops from pluripotent epithelial cells [3]. However, adenocarcinoma from remnants of t urachus remain is the most common [4]. Although adenocarcinoma appearing in remnant areas of the urachus differ clinically from those occurring at the base of the bladder, these neoplasms are similar in their histopathology and behaviour. Unlike tumors of the urachus, it is common to find in primary adenocarcinoma associated glandular cystitis. In our case a glandular metaplasia was present and suggests the first theory. The incidence of this cancer increases with age with peaks in the sixth, seventh and eighth decade [5].
Hematuria is the most common presenting symptom, accounting for approximately 90% of patients. Almost half of patients complain of dysuria, polakyuria, and pain. Our case also confirms these data [5]. Macroscopically, adenocarcinoma of bladder usually appear as simple nodular tumor that cannot be reliably distinguished from urothelial neoplasms.
Histologically, distinction between mucinous adenocarcinoma of bladder and carcinoma of urachus is difficult, as their presentations may be similar. The main clinical signs of adenocarcinoma of bladder are hematuria and dysuria, while mucusuria is observed in ~90% of urachial carcinomas. Wheeler and Hill proposed strict criteria for diagnosis of urachial adenocarcinoma: 1) seat in the dome 2) absence of cystic or glandular cystitis 3) infiltration of muscle and pelvic tissue with intact epithelium 4) Presence of urachal vestiges 5) Supra pubic mass 6) Clear demarcation between tumor and normal epithelium, and 7) Development of tumor in the wall of the bladder, with extension to Retzius space [6].
This distinction is crucial. First, treatment of urachal carcinoma is a partial cystectomy with a block resection of urachus rather than a radical cystectomy. In addition, urachal carcinoma has a better prognosis and a high survival rate compared to mucinous adenocarcinoma of bladder [6].
Differential diagnosis includes: Metastasis of a colon adenocarcinoma, prostatic metastasis, endometrial or ovarian metastasis in women, and urothelial neoplasms with glandular differentiation. Urothelial carcinoma with glandular differentiation may contain intracellular and luminal mucine; however, it is not abundant. Moreover, in this type of carcinoma, cells in kitten rings are not important and “glands” are surrounded by pseudostratified epithelium.
Differential diagnosis can also occur with non-neoplastic pathologies such as intestinal or nephrogenic metaplasia that can infiltrate the lamina propria or even the bladder wall. Mucous lakes are not uncommon in these cases and their presence in a biopsy should suggest the diagnosis of adenocarcinoma only if presence of neoplastic cells. Cells of intestinal metaplasia lack nuclear anaplasia and rarely involve the muscularis mucosa. Nodular areas of glandular cystitis rich on goblet cells should be considered benign even if nodules extend into the lamina propria. Immunohistochemistry is essential to confirm primacy and exclude other adenocarcinomas.
An immunohistochemical pannel positive for CK20, CDX2, and EMA but negative for PSA and CK7 is not suggestive of adenocarcinoma of the prostate but could correspond to an original bladder or colonic origin. Bladder adenocarcinoma is generally positive for CEA, CDX-2, MUC-1, MUC-2 and MUC-3, as well as metastatic colonic adenocarcinoma, and positive for both CK7 and CK20. In contrast, typical colonic adenocarcinoma has a negative CK7 and a positive CK20 profile [5]. In our case, it was a suggestive profile of a colonic origin first (CK20 positive, CK7 negative) but this profile has been reported in 29% of primary bladder adenocarcinomas too. We discard ovarian and endometrial origin given the absence of clinical and radiological signs in favor. This was confirmed after the radical treatment since histological examination showed the preservation of ovarian and gynecological structures in general.
We have seen previously the interest of research of a dysregulation of beta-catenine expression. Loss of nuclear expression of betacatenine plea, rather, for an urothelial origin. Note that these markers have no value in differentiating a primary bladder adenocarcinoma from a tumor of urachus.
Presence of variants with a similar immunohistochemical profile makes the diagnosis more difficult. Only by integrating clinical, radiological and immunohistochemical data is it possible to reach an accurate diagnosis from the beginning. Moreover, even when immunohistochemical study is correctly performed, because of the rarity of this tumor and histopathological difficulties encountered, unfortunately at the time of diagnosis about 46% of patients already have a stage IV tumor (Including lymph node positivity, T4b stage and distant metastasis) [6], requiring a palliative chemotherapy. In case of an operable tumor, a radical cystectomy represents the gold standard treatment. Prognostic factors for cancer includes stage of tumor, grade and subtype. According to a previous study, if cancer is limited to bladder, the survival rate can exceed 75% [7,8].
In European and American publications, all tumors are infiltrating >=T2, with a grade 2 or 3 [1,2,12]. In Egypt, however, in a serie of 185 cases, El-Mekresh reported 7% T1 tumors, and 52% grade 1 tumors.
In our experience, prognosis was favorable because patient had no distant metastasis or local spread of disease at the time of diagnosis. This was thanks to an early and adequate care [9].
Conclusion
Mucinous carcinoma of bladder presents a diagnostic dilemma for pathologists, given the common features with metastatic mucinous carcinoma. Certainly, immunohistochemistry becomes one of the essential actors in pathology however the confrontation with clinical and radiological data still keeps its place.
References
- Roy S and Parwani AV. Adenocarcinoma of the urinary bladder. Archives of Pathology & Laboratory Medicine. 2011; 135: 1601-1605.
- Grignon DJ, Ro JY, Ayala AG, Johnson DE, Ordonez NG. Primary adenocarcinoma of the urinary bladder. A clinicopathologic analysis of 72 cases. Cancer. 1991; 67: 2165-2172.
- Zhang BY, Aguilar J, Yang M, P.Wang, and Li B. Mucinousmetaplasia in urothelial tract may be the precancerous lesion of mucinous adenocarcinoma: Report of two cases and review of literature. International Journal of Clinical and Experimental Medicine. 2014; 7: 285-289.
- Mazzucchelli R, Scarpelli M, Montironi R. Mucinous adenocarcinoma with superficial stromal invasion and adenoma of urachal remnants: A case report. J Clin Pathol. 2003; 56: 465-467.
- Umesh D, Vishwanath S. A Case Report of Primary Mucinous Adenocarcinoma of Urinary Bladder with Review of Literature. American Journal of Clinical Medicine Research. 2014.
- Jasreman D, et al. Urachal carcinoma: A pathologic and clinical study of 46 cases. Hum Pathol. 2015; 46: 1808-1814.
- Tamboli P, Mohsin SK, Hailemariam S, Amin MB. Colonic adenocarcinoma metastatic to the urinary tract versus primary tumours of the urinary tract with glandular differentiation: A report of 7 cases and investigation using a limited immunohistochemical panel. Arch Pathol Lab Med. 2002; 126: 1057-1063.
- Bates AW and Baithun SI. Secondary neoplasms of the bladder are histological mimics of nontransitional cell primary tumours: Clinicopathological and histological features of 282 cases. Histopathology. 2000; 36: 32-40.
- El-Mekresh M, el-Baz M, Abol-Enein H, Ghoneim M. Primary adenocarcinoma of the urinary bladder: A report of 185 cases. Br J Urol. 1998; 82: 206-212.