Abstract
Background: A disintegrin and metalloproteinase (ADAM) 10 has been reported to be involved in certain autoimmune diseases, such as rheumatoid arthritis. In this study, we demonstrate that ADAM10, which is a member of the ADAM family, is expressed in angiitis, and we examine its relationship to this condition using clinical data.
Materials and Methods: The ADAM10 levels in serum from microscopic polyangiitis (MPA) patients and healthy controls were measured using enzymelinked immunosorbent assay. The clinical data were collected from Showa University cohort data. The relationship between the ADAM10 level in the sera and the clinical data was evaluated using Spearman’s rank correlation.
Results: No significant differences were observed in the mean age or gender ratio between the MPA patients and the healthy controls. The ADAM10 level in MPA serum (n=10) was significantly elevated compared with that in healthy control serum (n=7) (450 ± 44 pg/ml and 85 ± 33 pg/ml, respectively). The ADAM10 level in MPA serum was also significantly negatively correlated with myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA) titer, but not with the C-reactive protein level.
Discussion: ADAM10 is elevated in MPA patients and might be involved in their inflammation. On the other hand, ADAM10 is negatively correlated with MPO-ANCA. These results indicate that ADAM10 might be involved in inflammation other than MPO-ANCA.
Keywords: ADAM10; Angiitis; MPA; MPO-ANCA
Abbreviations
ADAMs: A Disintegrin and Metalloproteinases; MPA: Microscopic Polyangiitis; CRP: C-reactive Protein; MPO-ANCA: Myeloperoxidase Anti-Neutrophil Cytoplasmic Antibody
Introduction
A disintegrin and metalloproteinases (ADAMs) are a family of proteinases that are known to be involved in ectodomain cleavage and in the regulation of the intramembrane proteolysis of transmembrane proteins. ADAM10 and 17 are the major proteases that cleave some membrane-bound proteins, and have extensive overlap with and compensate for several substrates, including epidermal growth factor receptor ligands, tumor necrosis factor (TNF), TNF receptor, and interleukin (IL)-6 receptor [1,2]. ADAM17 is also known as TNF-α converting enzyme (TACE) and was identified as the primary protease responsible for the proteolytic processing of TNF-α. ADAM17 is expressed in numerous human tumors and is associated with invasion and metastasis [3,4]. ADAM10 is also involved in the shedding of many substrates that play roles in cancer progression, allergic responses and inflammatory diseases [5,6]. We previously showed that ADAM10 is involved in angiogenesis and the inflammation associated with rheumatoid arthritis (RA) [7,8]. However, the role of ADAM10 in other autoimmune diseases has not been examined. Here, we describe the expression and possible implications of ADAM10 in angiitis.
Materials and Methods
Patients
We used data from a cohort (n=10) of microscopic polyangiitis (MPA) patients (2009-2012). The sera were collected from the patients before their initial treatment with corticosteroids and/or immunosuppressants. Seven healthy subjects were recruited on a voluntary basis as controls. All the specimens were obtained with informed consent and were collected following approval from the Showa University Institutional Review Board. The participants completed the informed consent form by hand.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed as described previously [9]. The level of ADAM10 in serum was measured following the manufacturer’s protocol (My BioSource, San Diego, CA). The plates were developed using tetramethylbenzidine substrate (TMB, Sigma-Aldrich) and were read on a micro plate reader at 450nm.
Statistical analysis
The data were analyzed using Student’s t-tests assuming equal variances. The relationship between the ADAM10 level in sera and the clinical data was evaluated using Spearman’s rank correlation. The data are reported as the means ± SEM. P values less than 0.05 were considered statistically significant.
Results
Clinical characteristics of study subjects
The patient characteristics are summarized in Table 1. No significant differences were observed in the mean age or gender ratio between the MPA patients and the healthy controls.
MPA (n=10)
Healthy control (n=7)
p-value
Age
74 ± 1.6
65 ± 4.1
0.09
Female (%)
9 (90%)
6 (86%)
0.8
CRP (mg/dl)
5.0 ± 1.6
MPO-ANCA (IU/ml)
146 ± 49
Table 1: Summary of patient characteristics (mean ± SE).
ADAM10 in MPA serum
We measured the ADAM10 level in the serum from MPA patients and healthy controls using ELISA. We found that the ADAM10 level in MPA serum (n=10) was significantly elevated compared with that in healthy control serum (n=7) (450 ± 44 pg/ml and 85 ± 33 pg/ml, respectively, Figure 1A).
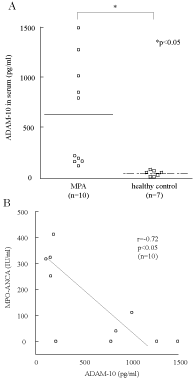
Figure 1: ADAM10 is expressed in the serum of MPA patients. A) The level
of ADAM10 in MPA patient serum is significantly higher than that in healthy
control serum; the p value is less than 0.05. B) The level of ADAM-10 in the
sera of MPA patients showed a significant negative correlation with MPOANCA
titer. (n=number of patients).
We then examined whether ADAM10 was related to the disease activity in MPA patients, particularly whether there was a relationship with the C-reactive protein (CRP) levels or myeloperoxidase antineutrophil cytoplasmic antibody (MPO-ANCA) levels. We found that the ADAM10 level in the serum from MPA patients was significantly negatively correlated with MPO-ANCA (Figure 1B). On the other hand, the ADAM10 level in MPA serum was not correlated with the CRP level. These results suggest that ADAM10 is involved in disease activity, but is not related to the presence of MPO-ANCA.
Discussion
We found that the ADAM10 level in serum is increased in MPA patients compared with healthy controls. However, the relationship between MPA and cytokine levels is still unclear. Berti et al. reported that the IL-6 serum level was significantly increased in patients with anti-neutrophil cytoplasmic auto antibodies associated vasculitides [10]. They also showed that tocilizumab (TCZ), which binds and inhibits the IL-6 receptor, induced a sustained disease remission in a patient with severe multi systemic MPA. We previously reported that ADAM10 is significantly positively correlated with disease activity in RA and is over expressed in the synovial tissues of RA patients [8]. We also showed that ADAM10 levels in RA serum were decreased after treatment with TCZ but not after treatment with adalimumab (an inhibitor of TNF-α) [11]. Interestingly, we found that the ADAM10 level in the serum of MPA patients was negatively correlated with MPO-ANCA titer, indicating that ADAM10 itself did not elicit the inflammation in MPA. Taken together, these findings suggest that ADAM10 might be involved in MPA via the regulation of IL-6.
We found that ADAM10 is negatively correlated with MPOANCA. Of note, some groups have previously reported that ANCA titers did not correlate well with disease activity, especially in MPO-ANCA disease [12,13]. Thai LH et al. also reported that the management of granulomatosis in patients with polyangiitis cannot be based on ANCA levels alone [14]. Our findings suggest that ADAM10 is another factor related to MPA disease activity.
In summary, we demonstrated that ADAM10 is elevated in the serum of MPA patients and might be involved in their inflammation. By contrast, ADAM10 is negatively correlated with MPO-ANCA titer. ADAM10 might be a predictive factor in MPA, in addition to MPO-ANCA.
References
- Garbers C, Janner N, Chalaris A, Moss ML, Floss DM, Meyer D, et al. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J Bio Chem. 2011; 286: 14804-14811.
- Folgosa L, Zellner HB, El Shikh ME, Conrad DH. Disturbed follicular architecture in B cell A disintegrin and metalloproteinase (ADAM) 10 knockouts is mediated by compensatory increases in ADAM17 and TNFalpha shedding. J Immunol. 2013; 191: 5951-5958.
- Ren J, Nie Y, Lv M, Shen S, Tang R, Xu Y, et al. Estrogen upregulates MICA/B expression in human non-small cell lung cancer through the regulation of ADAM17. Cell Mol Immunol. 2015; 12: 768-776.
- Park GB, Kim D. TLR4-mediated galectin-1 production triggers epithelialmesenchymal transition in colon cancer cells through ADAM10- and ADAM17-associated lactate production. Mol Cell Biochem. 2017; 425: 191- 202.
- Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, et al. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008; 102: 1192-1201.
- McCulloch DR, Akl P, Samaratunga H, Herington AC, Odorico DM. Expression of the disintegrin metalloprotease, ADAM-10, in prostate cancer and its regulation by dihydrotestosterone, insulin-like growth factor I, and epidermal growth factor in the prostate cancer cell model LNCaP. Clin Cancer Res. 2004; 10: 314-323.
- Isozaki T, Rabquer BJ, Ruth JH, Haines GK, 3rd, Koch AE. ADAM-10 is over expressed in rheumatoid arthritis synovial tissue and mediates angiogenesis. Arthritis Rheum. 2013; 65: 98-108.
- Isozaki T, Ishii S, Nishimi S, Nishimi A, Oguro N, Seki S, et al. A disintegrin and metalloprotease-10 is correlated with disease activity and mediates monocyte migration and adhesion in rheumatoid arthritis. Trans Res. 2015; 166: 244-253.
- Matsunawa M, Isozaki T, Odai T, Yajima N, Takeuchi HT, Negishi M, et al. Increased serum levels of soluble fractalkine (CX3CL1) correlate with disease activity in rheumatoid vasculitis. Arthritis Rheum. 2006; 54: 3408-3416.
- Berti A, Cavalli G, Campochiaro C, Guglielmi B, Baldissera E, Cappio S, et al. Interleukin-6 in ANCA-associated vasculitis: Rationale for successful treatment with tocilizumab. Semin Arthritis Rheum. 2015; 45: 48-54.
- Isozaki T, Nishimi S, Nishimi A, Saito M, Miwa Y, Toyoshima Y, et al. A disintegrin and metalloproteinase (ADAM)-10 as a predictive factor for tocilizumab effectiveness in rheumatoid arthritis. Mod Rheum. 2016: 1-5.
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Amer Soc Nephrol: JASN. 1996; 7: 23-32.
- Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum. 2008; 58: 2908-2918.
- Thai LH, Charles P, Resche-Rigon M, Desseaux K, Guillevin L. Are antiproteinase- 3 ANCA a useful marker of granulomatosis with polyangiitis (Wegener’s) relapses? Results of a retrospective study on 126 patients. Autoimmun Rev. 2014; 13: 313-318.