
Review Article
Austin J Autism & Relat Disabil. 2015;1(2): 1007.
QEEG and VARETA based Neurophysiological Indices of Brain Dysfunction in Attention Deficit and Autistic Spectrum Disorder
Robert J. Chabot¹, Robert Coben², Laurence Hirshberg³ and David S. Cantor4*
¹Department of Psychiatry, New York University School of Medicine, USA
²Department of Neuropsychology, Neurorehabilitation & Neuropsychological Services, USA
³Department of Psychiatry and Human Behavior, Brown University, USA
4Department of Mental Health Services, Mind & Motion Developmental Centers of Georgia, USA
*Corresponding author: David S. Cantor, Department of Mental Health Services, Mind & Motion Developmental Centers of Georgia, 5050 Research Ct, Ste 800, Suwanee, GA 30045, USA
Received: September 26, 2014; Accepted: May 20, 2015; Published: May 25, 2015
Abstract
Quantitative Electroencephalogram (QEEG) and EEG source localization were used to describe the patho-physiological nature of brain dysfunction in children with Attention Deficit Hyperactivity Disorder (ADHD) or Autism Spectrum Disorder (ASD). QEEG frequency analyses revealed 4 subtypes that differed both in severity of abnormality and relative frequency of occurrence in both disorders but do not clarify distinctive neural networks associated within each of the disorders. Multivariate discriminant analyses proved to be effective in discriminating clinical groups from normal and from each other with high levels of sensitivity and specificity. EEG source localization indicted that ADHD was characterized by functional abnormality within the thalamus, hippocampus, caudate nucleus, and anterior cingulate, frontal/striatal, temporal, and parietal regions bilaterally and ASD by functional abnormality within the thalamus, hippocampus, caudate nucleus, and posterior cingulate, supramarginal gyrus, lateral and medial occipital/temporal, superior parietal, and occipital cortical regions bilaterally.
Keywords: Autism; ADHD; QEEG; VARETA; LORETA; Neurophysiology
Background
Attention Deficit Hyperactivity Disorder (ADHD) and Autistic Spectrum Disorder (ASD) are two neurodevelopmental disorders which at various times in the past 40 years have been described as being epidemic in their scale amongst childhood psychiatric disorders. Both disorders occur early in childhood and can have extreme effects on the lives of these children. ADHD is characterized by symptoms of inattention, impulsive behavior, and varying degrees of hyperactivity which often result in problems in learning, cognition, and social interactions. Autistic spectrum disorder is characterized by deficits in social interaction and communication often accompanied by repetitive behavior and dysfunction in executive function, language, and emotional behavior. ASD individuals can also exhibit impaired attention regulation processes such as distractibility or at other times significant problems with hyper-focusing and difficulty in shifting their attention as needed. While several recent studies have documented the neuro-physiological and neuro-anatomical nature of these disorders [1,2], there are no published studies that examine the similarities and differences between the brain structures and neurological functions compromised within each if these disorders and a set of heuristics that can help provide the discriminability of these disorders
Quantitative EEG (QEEG) is a valuable technique used in the diagnosis and treatment of children and adults with psychiatric and neurological disorders [3,4]. The clinical utility of QEEG in child and adolescent psychiatric disorders including autism, specific developmental disorders, and attention deficit disorder has been documented [5]. QEEG is useful for aiding in the differential diagnosis of children with learning disorders and those with various subtypes of attention deficit disorder [1].
Variable Resolution Electromagnetic Tomography (VARETA) is a 3 dimensional source localization method that uses surface recorded EEG to analyze current density and to identify the most probable neuro-anatomical generators of each EEG frequency band. The results of these analyses can be used to generate maps based upon a probabilistic brain atlas resembling slices obtained from a Magnetic Resonance Image (MRI) [6]. When z-score transformed relative to a normal population these VARETA brain images can be used to depict the cortical and sub-cortical structures involved in the pathophysiology of various neuro-cognitive disorders. The VARETA technique has been shown to be useful in the identification of the neuro-anatomical structures involved in; (1) epileptic activity generation [7,8], (2) hypoperfused regions due to neurocysticercosis, reversible ischemic attacks, and cerebral artery disease [6,9,10], (3) space occupying lesions [11,12], (4) the localization of cognitive processes [13], obsessive-compulsive disorder [14], and attention deficit disorder [15].
The present study was designed to document QEEG differences between large samples of children diagnosed with ASD, ADHD, and a matched sample of children with no known neurological or psychiatric disorders. The goal was to document the specific types of QEEG profiles found within these populations and to develop QEEG feature based discriminant functions (possible biomarkers) to distinguish children with ASD and those with ADHD from the normal population of children as well as from each other. VARETA was utilized to identify the neuro-anatomical structures that underlie the pathophysiology of the childhood syndromes of ASD and ADHD and to identify any neurophysiological subtypes that exist within each disorder. We also identify the cortical and subcortical regions that generate abnormal activity within each disorder and then localize the anatomical differences between the two disorders. Collaborative evidence supporting our findings will be provided by reviewing the findings from brain structural imaging studies (MRI, fMRI, PET) of these two disorders.
Method
Normal population
A sample of 92 normal children from the NYU database of normal children was selected to match the age and sex distributions of our sample of ASD and ADHD children. All normal subjects were free of neurological or medical disease, had no history of head injury, drug or alcohol abuse, were of normal IQ, showed evidence of adequate functioning at home/school for the past two years, and had not taken any prescription medication for at least 90 days prior to evaluation. Specific details of the procedures used to establish the normal data base have been previously published [16]. The reliability of this normal data base has been validated using independent samples of normal individuals [17-22]. This replication of the age-regression equations developed on the above data base justifies their generalized application [23].
Clinical populations
All Autistic Spectrum Disordered (ASD) children used in this study were referred to the Neurodevelopment Center in Providence Rhode Island or the Neurorehabilitation and Neuropsychological Center in Massapequa, New York. All ADHD children were referred to the Developmental Pediatrics and Learning Disorders Clinic in Sydney, Australia. Samples of 92 children were entered into this study from each of these clinical groups. All children were examined by a neuropsychologist and had a neuropsychological and QEEG evaluation. Children with histories of epilepsy, drug abuse, head injury, or psychotic disorders were excluded. The clinical and neuropsychological evaluations obtained on each child were those tests routinely administered at each outpatient clinic. None of children used in this sample, were on any medications.
The demographic information from these samples is shown in Table 1 indicating no significant differences between groups in terms of age or sex. None of the children used in this sample were on medication at the time of QEEG testing. An additional 14 ASD children had QEEG evaluations while on medication and these children were used to test for general medication effects on the QEEG.
Normal Kids
ASD
F value
P value
Mean
(SD)
Range
Mean
(SD)
Range
Age: 9.98 (2.98) 6-17
10.01
3.3
6-17
10.03
3.1
6-17
0.1
<.99
VIQ: WNL
97.0
9.4
73-118
90.9
18.0
44-136
3.5
<.06
PIQ: WNL
97.8
9.5
74-126
92.7
17.0
46-136
16.2
<.0001
IQ: WNL
99.2
9.7
85-127
89.8
15.6
53-130
14.4
<.0002
Sex
92 males; 13 females
92 males; 13 females
92 males; 13 females
Table 1: Table showing findings for matching samples on age, sex and IQ.
Quantitative EEG (QEEG) methodology
The neurometric method of QEEG data collection and analysis was utilized. The EEG power at each frequency, recorded from 19 electrodes located on the scalp in compliance with internationally standardized procedures, is subjected to visual editing to remove artifactual contamination by non-cerebral sources such as movements and then subjected to computer analysis to extract a wide variety of descriptive measures. The measures are grouped by broad frequency bands, into which the EEG is conventionally divided: delta (1.5-3.5 Hz), theta (3.5-7.5 Hz), alpha (7.5-12.5 Hz), and beta (12.5-25 Hz). For each broad band frequency band, univariate measures were derived for: 1) absolute power; 2) relative power; 3) interhemispheric and intrahemispheric power asymmetry; and 4) interhemispheric and intrahemispheric coherence. Additionally, measures for various multivariate regional absolute power, relative power, power asymmetry, and coherence measures across the frequency spectrum; and multivariate measures collapsed across all frequency ranges were derived. The interested reader is referred to several published papers for a detailed description of this technique and the development of the normal EEG database [22,24,25].
All ADHD and ASD children were seated comfortably in a sound and light attenuated room during the evaluation. Electrode caps were used to place recording electrodes over the 19 standard regions defined by the International 10/20 system referenced to linked ears. All electrode impedance levels were kept below 5000 ohms. Twenty to thirty minutes of continuous eyes closed resting EEG was recorded using a Spectrum 32 (ADHD) or Deymed (ASD) EEG system. An experienced EEG technician observed the continuous EEG being recorded and selected from 1 to 2 minutes of artifact-free EEG for further analysis. All EEG was digitized and placed onto CDs for entry into a computer for subsequent quantification. Prior to EEG quantification, all EEG was examined by one of the authors who removed artifact contaminated epochs missed by the first technician. Particular care was taken to prevent EEG contamination due to drowsiness, and to exclude EEG segments contaminated by horizontal and lateral eye-movement, muscle activity, ECG artifact, or by EEG transients due to sharp waves or paroxysmal activity. EEG quantification was restricted to those children from whom a minimum of one minute of artifact-free EEG (24 epochs) could be obtained. Prior research has shown that this is the minimum amount of EEG required to obtain reliable quantitative EEG measures [26]. The artifact free EEG segments were read into the Nxlink software for quantification. This software converts the Deymed and Spectrum 32 EEG segments to meet the amplifier and frequency characteristics under which the normal database was collected and to equivocate digitized information collected from the different amplifier systems. The artifact-free EEG from each channel was then converted from the time to the frequency domain via Fast Fourier Transform (FFT). Each QEEG measure was compared to the mean and standard deviation of that measure obtained from the age-regressed normal database using a Z or standard score.
The VARETA technique
In the last few years a new method for localizing electrical activity in the brain, called Variable Resolution Electromagnetic Tomography (VARETA), has been developed. This imaging technique allows an estimation of the distribution of the electrical generators for each frequency band within the brain, by applying a mathematical inverse solution to the EEG data. The anatomical definitions of regional probability for source localization used in VARETA are derived from a Probabilistic Brain Atlas (PBA) developed at the Montreal Neurological Institute [27]. Use of the PBA obviates the need for individual MRI scans, in exchange for sacrificing precise anatomical localization. Three-dimensional coordinates for the position of each scalp electrode position, defined by the proportional 10/20 International Electrode Placement System, have been published [28]. These coordinates were used to project each electrode position onto the average surface of the mean dimensions of an age appropriate head, thus placing the proportional EEG electrode set into spatial registration with the proportional PBA. Based on this EEG-MRI head model, the problem of the 3-D sources of EEG may be specified in the frequency domain [6,29,30]. Resting, eyes closed EEGs from the normal population, constituted a normative database for VARETA using narrow band spectral analysis between 0.39 Hz to 19 Hz in increments of 0.39 Hz [31]. Using the resulting set of normal values for narrow band spectral power at each scalp electrode [29,32], the sources of power at each frequency were localized. Three-dimensional color-coded tomographic images were then generated, with source generator distributions superimposed upon the transaxial, coronal, and sagittal slices color-coded as z-score deviations from the normal population. The VARETA method is a useful adjunct to QEEG analysis. It provides additional information about the localization of the abnormal sources of surface electrical activity. Nevertheless, VARETA, like other inverse methods, presents some limitations: it represents an approximate solution for the most probable neuroanatomical generators. However studies have demonstrated the spatial resolution of such imaging techniques is with 5 mm consistent with MRI imaging for specific locations.
Results
QEEG discriminant analysis findings
A series of step-wise discriminant analyses were calculated comparing the normal children with ASD children, the normal children with the children with ADHD, and the ASD and ADHD children with each other. QEEG variables entered were selected using analysis of variance comparisons between the two groups with those variables with the highest F-ratios and lowest inter-correlations chosen. A total of 5 variables were used to discriminate the normal from the ADHD children with a sensitivity of 93.3%. A specificity of 88.6% with Positive Predictive Validity (PPV) of 89.1% and Negative Predictive Validity (NPV) of 93.0%. Variables utilized included delta and theta relative power and frontal coherence measures. A total of 5 variables were utilized to discriminate the normal from the ASD children with 92.3% sensitivity and 95.6% specificity. The positive predictive value was 95.5% and the negative predictive value was 92.6%. QEEG variables utilized included delta mean frequency, theta coherence, delta relative power, frontal/temporal alpha asymmetry, and frontal/temporal theta relative power. A total of 4 variables were used to discriminate the ASD from the ADHD children with 82.9% sensitivity, 79.0% specificity and PPV of 82.0% and NPV of 79.0%. Variables utilized included measures of delta absolute power, frontal theta coherence, and alpha temporal asymmetry and frontal/ posterior theta asymmetry.
QEEG subtypes
In processing the ADHD and ASD data, we examined the narrow band spectral analyses from .39 to 19 Hz for each child across all 19 electrode placements and identified the frequency at which the maximum deviation from normal occurred. We then used these narrow band spectral results to place the ASD and ADHD children separately into 4 sub-types or phenotypes to include those with maximal narrow band spectral deviations from normal that fell between; (1) 3.9-6.63 Hz, (2) 7.02-8.58 Hz, (3) 8.97-10.53 Hz, and (4) 10.92-14.04 Hz. Results from these analyses are listed in Table 2.
Cluster Subtypes of significant (p<.10) QEEG variable deviations*
Clinical Group
Cluster 1
Cluster 2
Cluster 3
Cluster 4
ADHD
¯ Generalized Delta
↑ Generalized Theta
↑Central & Temporal Beta
¯ Generalized Delta
↑ Generalized Theta (esp Frontal)
↑ Generalized Alpha (esp Central)
↓Posterior relative pwr Beta
¯ Generalized Delta
↑ Frontal Theta
↑Generalized Alpha
↓Posterior Beta
¯ Frontal and Central Delta
↑ Frontal Theta
↑Frontal and Central Beta
Representative %
17.5%
25.5%
24.1%
13.9%
ASD
↓Generalized Delta
↑Generalized Theta
↓Posterior Alpha
↑Frontal Beta
↓Generalized Delta
↑Generalized Theta
↑Generalized Alpha (esp Frontal and Central)
↓Posterior Beta
↓Generalized Delta
↓Central and Posterior Theta
↑Generalized Alpha
↓General Delta
↑Frontal and Central Alpha
↑Generalized Beta
Representative %
15.3%
17.5%
23.4%
24.1%
*All measures were of absolute power unless otherwise specified
Note that a common subtype characteristic across all clusters for both clinical populations is the presence of Delta deficits.
Table 2: Table showing a summary of the qEEG cluster types, their representation in the clinical groups, and the relative trends in Z-scores in these clusters for each clinical group.
For ADHD, these subtypes included 17.5%, 25.5%, 24.1%, and 13.9% of these children respectively. For ASD, these subtypes included 15.3%, 17.5%, 23.4%, and 24.1% of these children respectively. While the distributions of these EEG frequency based subtypes were similar for both developmental disorders, more ADHD children showed abnormality between 7 and 9 Hz (high theta and low alpha) and more ASD children had abnormal findings between 11 to 14 Hz activities (beta). In addition, 19.0% of the ADHD and 19.7% of the ASD children had narrow band spectral analysis which failed to show a clear cut maximum peak deviation from normal and these children were not included within the present analyses. Group average VARETA images were then constructed for each of the 4 above defined subtypes separately for the ADHD and ASD children. Analysis of variance ‘F’ ratios were calculated comparing the VARETA images of the ADHD and ASD children at each of the 4 narrow band frequency groupings.
QEEG subtype differences
In order to document the QEEG differences between each of the ADHD and ASD subtypes defined in this study on the basis of the narrow spectral band findings described above, we calculated group average head maps of z-score relative power across the traditional delta, theta, alpha, and beta frequency bands. This makes the present work more amenable for comparison with other published studies which utilize these frequency bands. Figure 1 presents these head maps separately for the ADHD and ASD children with each grouping representing a neurophysiological subtype of each disorder based upon frequency distribution differences. Table 2 summarizes these general subtypes for power measures exceeding p< .10 in tabular form.
It was found that the EEGs of an independent sample of 14 medicated ASD children were distributed across all subtypes. ANOVAs computed comparing relative power findings between the medicated and non-medicated children indicated that medication resulted in less of a delta relative power deficit (p< .01), especially in frontal and central regions, and an increase in frontal alpha (p< .01) and beta (p< .03) relative power.
VARETA subtypes
Figures 2,3,4, and 5 present the group average VARETA images in the axial plane for each narrow frequency band grouped separately for the ADHD and ASD children as well as the analysis of variance F-values for the significance of the difference between the ADHD and ASD groups. All VARETA images use threshold scaling such that colors shown represent statistically significant deviations from the normal population (upper two panels of each figure) or significant ANOVA differences between the ADHD and ASD children (bottom panel each figure). Note that the anatomical location of abnormal neurophysiological activity is very consistent across the ADHD and ASD subtypes with greater differences seen when comparing each ADHD and ASD subtype against each other. Consistent differences were seen between the ADHD and ASD subtypes at each frequency and these differences showed virtually the same pattern of anatomical abnormality across subtypes. In other words, despite the different frequency distributions noted between ADHD and ASD subtypes (as demonstrated in Figure 1; Table 2), the neuroanatomical structures identified by VARETA as showing abnormal activity are consistent within both the ADHD and ASD populations which differ significantly from one another.
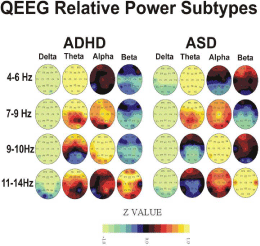
Figure 1: Figure showing univariate Z-score brain maps for a variety of broad
bands of the qEEG.
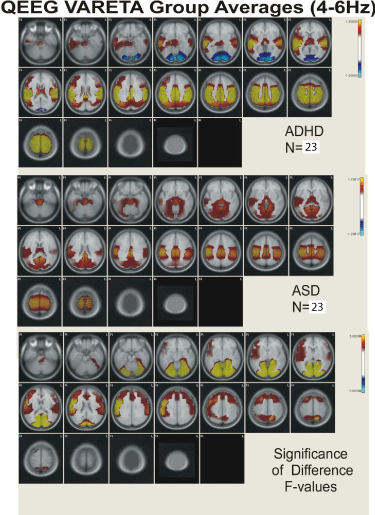
Figure 2: Figure showing VARETA structures and corresponding Z-scores in
current density for the broad band 4-6 Hz activity.
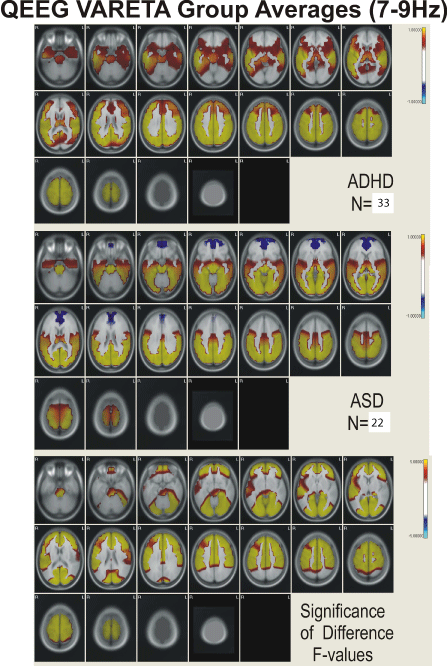
Figure 3: Figure showing VARETA structures and corresponding Z-scores in
current density for the broad band 7-9 Hz activity.
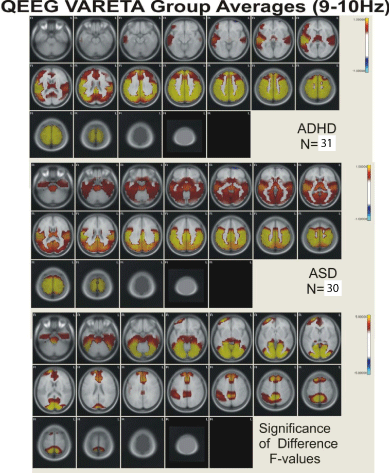
Figure 4: Figure showing VARETA structures and corresponding Z-scores in
current density for the broad band 9-10 Hz activity.
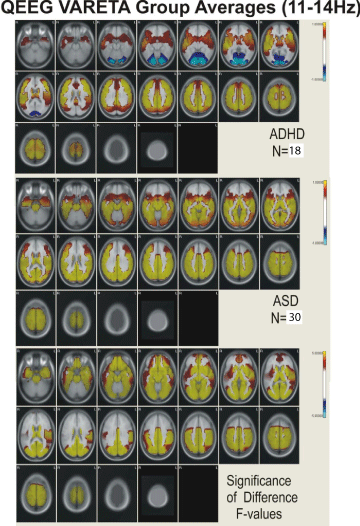
Figure 5: Figure showing VARETA structures and corresponding Z-scores in
current density for the broad band 11-14 Hz activity.
Table 3 presents a comparison of the anatomical regions showing abnormal neurophysiological activity for the ADHD and ASD children. In general, ADHD is characterized by abnormal increased neurophysiological activity in the thalamus, caudate nucleus, cingulate, and in frontal, temporal, precentral, postcentral, parietal, and occipital cortical regions with decreased activity in the cerebellum. ASD children were characterized by increased activity in the cerebellum, thalamus, hippocampus, in parahippocampal, cuneus, cingulate, and lingual gyri, and in temporal, precentral, postcentral, parietal, and occipital cortical regions. In ADHD, abnormal activity was greater in inferior and superior frontal regions, in precentral and postcentral cortical regions, and in anterior cingulate cortex and frontal/striatal regions than in ASD. In ASD, abnormal activity was greater than in ADHD in cerebellum, parahippocamus, lingual and parahippocampal gyri, and in occipital/temporal and posterior cingulate cortical regions.
SubCortical & Cortical
Structures showing VARETA Abnormality
Attention Deficit Disorder
Autistic Spectrum Disorder
Significant Differences
Cerebellum
X
X
ADHD less
Thalamus
X
X
None
Caudate
X
X
None
Hippocampus
X
X
None
Inf. Mid. Sup. Temporal
ALL
Mid & Sup
ASD less
Inf. Mid. Sup. Frontal
ALL
Mid
ASD less
Precentral Gyrus
X
ASD less
Postcentral Gyrus
X
X
None
Inf. Mid. Sup. Occipital
X
Superior
ADHD less
Lat Mid Occipital/Temporal
X
ADHD less
Sup. Parietal
X
X
ADHD less
Angular Gyrus
X
X
None
Supramarginal Gyrus
X
ADHD less
Cuneus
X
X
None
Lingual Gyrus
X
N
None
Cingulate
Anterior & Posterior
Posterior
None
Table 3: Brain Structures Showing Abnormal Function using VARETA in Children with Attention Deficit Disorder and Autistic Spectrum Disorder.
Discussion
The majority of children with attention deficit disorder and those with ASD show QEEG abnormality indicative of functional impairment at sub-cortical and cortical regions. When very narrow band z-scored spectral components are examined within these two populations of children, 4 major subtypes or phenotypes of QEEG abnormality are identified and these were illustrated using the traditional broad band spectral components of z-relative power in Figure 1 and Table 2. When examining these findings, the commonalities of the QEEG features found in both the ADHD and ASD subgroups include generalized decreased delta activity and increased generalized theta activity. While some children from both the ADHD and ASD population fall into each of the four subtypes; theta excess, theta and alpha excess, alpha excess, and beta excess, the percentages of children within each subtype and the overall nature of each subtype varies across diagnostic category. A greater percentage of the ADHD children fell within the theta and alpha excess subtype (25.5% vs. 17.5%) while more ASD children showed a beta excess (24.1% vs. 13.8%). The ADHD children within all subtypes showed increased frontal theta not seen in the ASD children, and the degree of delta deficit present within each subtype was greater for the ASD population which also showed a greater degree of beta excess across subtypes than did the ADHD children. This beta excess is partially due to medication effects although the finding of a greater beta excess in ASD holds true when the medicated children were removed from all analyses. The beta excess present is noteworthy and was present with or without the presence of medication. The increased frontal abnormality in ADHD may reflect frontal/striatal dysfunction and the disruption of executive function often associated with problems of attention [33]. The deficit of delta common in ASD may reflect the cortical and subcortical connectivity issues often described in ASD [34]. The delta EEG rhythm has been hypothesized to play a role as an integrative mechanism across brain regions with two thalamo/ cortical networks active. The first network involves the thalamus and its’ connections to specific cortical regions and the second, involving delta, as a global integrative network [35]. In fact, Alper [36] suggests that delta power is modulated by dopamine and acts by facilitating the transition between local and global brain states.
Clarke [37] used cluster analysis of QEEG to document the existence of three ADHD subtypes in a sample of 184 ADHD boys and 40 age and gender-matched controls. Subtype 1 showed increased total power, increased relative theta and decreased relative delta and beta waves, and type 2 showed increased relative theta and decreased relative alpha and increased central/posterior relative delta. The third subtype showed increased relative beta and decreased relative alpha activity. These findings are similar to those reported in the present paper for our ADHD population. These findings are also in agreement with previous studies of eyes-closed resting QEEG in 407 children with ADHD or ADD. In these studies, QEEG frequency abnormality occurred in over 80% of these children with theta and alpha excess the most prevalent abnormal finding. Frontal and central regions were most likely to be involved, and if generalized, the magnitude of the frequency abnormality was greatest in these regions [38-40].
Studies of EEG frequency abnormality in children with ASD have provided less consistent results over those seen in ADHD. For example, two studies showed decreased delta frontally [2,41], while one found increased activity in the delta range [42]. Two studies reported increased generalized delta or described “slowing” [43,44]. Three studies showed theta increases [2,42,45], while one study reported reduced theta [41]. These inconsistencies do not appear to be accounted for by considering whether the study was done with the subjects in a resting state or under task conditions. By contrast, findings have been quite consistent with spectral power analyses of faster frequencies, alpha through gamma. All studies that completed spectral power analyses reported reduced alpha power [41,43] and increased beta [2,42,46,47] and gamma power [48]. These inconsistent results are possibly due to effects of early use of psychotropic medications on brain-behavior development [5] even when the subjects at the time of study were off medication and/or due to the limited sample sizes utilized and may also reflect the heterogeneity found in this population of children. In the present study with substantially larger samples of ASD children this heterogeneity is substantiated by the existence of at least 4 subtypes of QEEG frequency abnormality which encompass most of the findings described above.
The results of the VARETA analyses suggests that despite different patterns of EEG frequency abnormality across ADHD and ASD children, abnormalities occur in specific regions of interest between ADHD and ASD children which are noteworthy. More specifically, abnormalities are noted in the Thalamus, Caudate, Hippocampus, Post-Central Gyrus, Angular Gyrus, Cuneus, and Lingual Gyrus. The co-occurring abnormalities in these common structures likely accounts for many of the commonalities found in these two clinical populations. There appears to be a single underlying neurophysiological pathway or network that can be identified within each disorder. When processed using the VARETA software all four QEEG subtypes within a diagnostic category showed similar patterns of sub-cortical and cortical abnormality with consistent differences between the ADHD and ASD children present for each subtype. VARETA images of ADHD children revealed functional abnormality within the thalamus, hippocampus, and caudate nucleus that spread to and included the anterior cingulate, frontal/striatal, temporal, and parietal regions bilaterally. VARETA images of ASD children revealed functional abnormality within the thalamus, hippocampus, and caudate nucleus that spread to and included the posterior cingulate, supramarginal gyrus, lateral and medial occipital/temporal, superior parietal, and occipital cortical regions bilaterally. The sub-cortical and cortical regions showing abnormal neurophysiological function in ADHD and ASD children identified using QEEG based VARETA imaging agrees with the findings based upon other neuroimaging techniques such as MRI, fMRI, and PET.
Neuroimaging studies of ADHD indicate decreased regulation of the cerebellum and the frontal/striatal system [49] and decreased activation of frontal regions and connections between bilateral prefrontal regions and the temporal and parietal cortices, regions important for cognitive flexibility and executive function [33,50,51]. Disruptions in function of the frontal\striatal system and its connections with the caudate nucleus have also been reported [52]. Decreased activation of bilateral parietal regions, the precuneus region and the thalamus may indicate disturbances in salient feature detection and the ability to shift attention deficits often characteristic of ADHD [53]. Significant disturbances in the connections between the anterior cingulate cortex, the precuneus region and prefrontal cortex and with the posterior cingulate cortex have been reported in ADHD [54]. In fact, anterior cingulate cortex function has been shown to play a role in the executive control of attention another area of attention processing in which those with ADHD often have problems [55].
In contrast, neuroimaging studies of ASD suggest abnormal function between frontal/striatal systems and more posterior cortical regions. This involves the disruption of the frontal/striatal and parietal networks important in the social brain system [56], and disruption of communication between the frontal/striatal, cerebellum, basal ganglia, thalamus, and ventral striatum important in mental state attribution and the superior temporal region important in perception and eye gaze [57], and decreased grey matter in frontal/ temporal and somatosensory regions involved in social cognition [58]. Further studies in ASD note disruption of the connections between the posterior cingulate region and the inferior and ventral temporal regions involved with the integration of visual and affective information [59], decreased activity in the superior temporal region and the cerebellum involved in the integration of sensory and limbic information and social perceptual skills [60], and decreased caudate nucleus volume and repetitive behavior [61].
The sub-cortical and cortical regions showing abnormal neurophysiological function in ADHD and ASD children identified using QEEG based VARETA imaging is supported by the findings described above which were based upon other neuroimaging techniques such as MRI, fMRI, and PET. Clearly, both QEEG and VARETA can play an important role in identifying the underlying physiological abnormality present in both ADHD and ASD. Individual patterns of findings may have implications for diagnostic purposes as well as for treatment selection and implementation. For example individual QEEG frequency profiles can be used to guide Neurofeedback to reduce the salient QEEG/VARETA abnormalities. Such training protocols have recently shown to have promise in ADHD and ASD [62,63]. With the identification of more specific network involvement in each of these populations, neurofeedback targeting structures using VARETA or LORETA (Low Resolution Electromagnetic Tomographic Analyses) may have promise for more potent means of achieving clinical improvement [64]. Neuropharmacotherapy can also use various pharmacological agents guided and assayed by their ability to normalize the QEEG which, from other pharmacokinetic studies, will predict favorable clinical responses [65].
References
- Chabot RJ, di Michele F, Prichep LS. The role of quantitative electroencephalography in child and adolescent psychiatric disorders. Child and Adolescent Psychiatric Clinics of North America. 2005; 14: 21-53.
- Coben R, Clarke AR, Hudspeth W, Barry RJ. EEG power and coherence in autistic spectrum disorder. Clinical Neurophysiology. 2008; 119: 1002-1009.
- Coburn KA, Lauterbach EL, Boutros N, Arciniegas D, Black K, Coffey E. The value of Quantitative electroencephalography in Clinical Psychiatry. Journal of Neuropsychiatry and Clinical Neurosciences. 2006; 18: 460-500.
- Hughes JR, John ER. Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci. 1999; 11: 190-208.
- Cantor D, Malone M, ChabotR. QEEG and VARETA Profiles in Autism Spectrum Disorders: Severity and Medication Correlates. Presentation at the 37th Annual International Neuropsychological Society Conference, Atlanta, GA, Sept. 2009; 11-14.
- Bosch-Bayard J, Valdes-Sosa P, Virues-Alba E, Aubert-Vazquez E, John ER, Harmony T, et al. 3D Statistical Parametric Mapping of EEG Source Spectra by means of Variable Resolution Electromagnetic Tomography (VARETA). Clin EEG. 2001; 32: 47-61.
- Morales-Chacon LM, Bosch-Bayard J, Bender-del Busto JE, Garcia-Maeso I, Galan-Garcia L. [Video-EEG evaluation complemented by spectral and EEG source analysis in patients with medication-resistant medial temporal lobe epilepsy]. Rev Neurol. 2007; 44: 139-145.
- Santiago-Rodriguez E, Harmony T, Fernandez-Bouzas A, Hernandez-Balderas A, Martinez-Lopez M, Graef A, et al . Source analysis of polyspike and wave complexes in juvenile myoclonic epilepsy. Seizure. 2002; 11: 320-324.
- Fernandez-Bouzas A , Harmony T, Fernandez T, Ricardo-Garcell J, Casian G, Sanchez-Conde R. Cerebral blood flow and sources of abnormal EEG activity (VARETA) in neurocysticercosis. Clinical Neurophysiology. 2001; 112: 2281-2287.
- Fernandez-Bouzas A, Harmony T , Fernandez T, Ricardo-Garcell J, Santiago E. Variable resolution electromagnetic tomography (VARETA) in evaluation of compression of cerebral arteries due to deep midline brain lesions. Archives of Medical Research. 2004; 35: 225-230.
- Prichep LS, John ER, Tom M. Localization of deep white matter lymphoma using VARETA - A case study. Clinical EEG. 2001; 32: 62-66.
- Fernandez-Bouzas A, Harmony T, Bosch JAE, Fernandez T, Valdes P, Silva J, et al. Sources of abnormal EEG activity in the presence of brain lesions. Clin Electroencephalogr. 1999; 30: 46-52.
- Harmony T, Fernández T, Gersenowies J, Galan L, Fernandez-Bouzas A, Aubert A, Diaz-Comas L. Specific EEG frequencies signal general common cognitive processes as well as specific task processes in man . International Journal of Psychophysiology. 2004; 53: 207-216.
- Bolwig TG, Hansen ES, Hansen A, Merkin H, Prichep LS. Toward a better understanding of the pathophysiology of OCD SSRI responder: QEEG source localization. Acta Psychiatr Scand. 2007; 115: 237-242.
- di Michele F, Prichep LS, John ER, Chabot RJ. The neurophysiology of attention-deficit/hyperactivity disorder. International Journal of Psychophysiology. 2005; 58: 81-93.
- John ER, Ahn H, Prichep LS, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980; 210: 1255-1258.
- Alvarez A, Sosa V, Marqui V, Garcia G, Biscay L, Bayard B. 1989. On the structure of EEG development. Clin. Neurophysiol. 1989; 73: 10-19.
- Alvarez A, Pascual R, Valdes P. EEG development equations confirmed for Cuban school children. Electroenceph Clin Neurophysiol. 1987; 67: 330-332.
- Gasser T, Mochs J, Lennard HJ, Bacher P, Verleger R. The EEG of mildly retarded Children: Developmental, classificatory and topographic aspects. EEG Clin Neurophysiol. 1983; 55: 131-144.
- Harmony T. Psychophysiological evaluation of children's neuropsychological disorders. 1988; 265-290.
- Jonkman EJ, Poortvliet DCJ, Veering MM, DeWeerd AW, John ER. The use of neurometrics in the study of patients with cerebral ischemia. Electroencephalogr Clin Neurophysiol. 1985; 61: 333-341.
- Ritchlin CT, Chabot RJ, Alper K, Buyon J, Belmont HM, Roubey R, et al. Quantitative electroencephalography: A new approach to the diagnosis of cerebral dysfunction in systemic lupus erythematosus. Arthiritis & Rheumatism. 1992; 35: 1330-1342.
- Lopes Da Silva F. A critical review of clinical applications of topographic mapping of brain potentials. J. Clin Neurophysiol. 1990; 7: 535-551.
- John ER, Prichep LS, Ahn H, Easton P, Fridman J, Kaye H. Neurometric evaluation of cognitive dysfunctions and neurological disorders in children. Progress in Neurobiology. 1983; 21: 239-290.
- John ER, Prichep LS, Friedman J, Easton P. Neurometrics: Computer-assisted differential diagnosis of brain dysfunctions. Science. 1988; 239: 162-169.
- John ER, Prichep LS. Normative development equations for the EEG and their sensitivity to neurological and psychiatric disorders. In Electric and Magnetic Activity of the Central Nervous System: Research and Clinical Applications in Aerospace Medicine. 1987; 7: 1-3.
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes. Proceedings of IEEE-Nuclear Science Symposium and Medical Imaging Conference. 1993; 95: 1813-1817.
- Scherg M, Von Cramon D. Two bilateral sources of the late AEP as identified by a spatio-temporal dipole model. EEG Clin Neurophysiol. 1985; 62: 32-44.
- Valdes-Sosa P, Bosch J, Gray F, Hernandez J, Riera J, Pascual R, et al. Frequency domain models of the EEG. Brain Topog. 1992; 4: 309-319.
- Valdes-Sosa P, Marti F, Garcia F, Casanova R. Variable resolution electromagnetic tomography. In Proceedings of the 10th International Conference on Biomagnetism: Biomag '96, edn. Wood C, Santa Fe, New Mexico: 1996.
- John ER, Karmel BZ, Corning WC, Easton P, Brown D, Ahn H, et al. Neurometrics: Numerical taxonomy identifies different profiles of brain functions within groups of behaviorally similar people. Science. 1977; 196: 1393-1410.
- Valdes-Sosa P, Biscay R, Galan L, Bosch J, Szava S, Virues T. High resolution spectral EEG norms for topography. Brain Topography. 1990; 3: 281-282.
- Carrey NJ, MacMaster FP, Gaudet L, Schmidt MH. Striatal Creatine and Glutamate/Glutamine in Attention-Deficit/Hyperactivity Disorder. Journal of Child and Adolescent Psychopharmacology. 2007; 17: 11-17.
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long distance disconnection. Current opinion in Neurobiology. 2005; 15: 225-230.
- Evans BM. Sleep, consciousness and the spontaneous and evoked electrical activity of the brain. Is there a cortical integrating mechanism? Neurophysiol Clin. 2003; 33: 1-10.
- Alper KR. The EEG and cocaine sensitization: A hypothesis. J Neuropsychiat Clin Neurosci. 1999; 11; 209-221.
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clinical Neurophysiology. 2001; 112: 2098-2105.
- Chabot RJ, Serfontein G. Quantitative Electroencephalographic Profiles of Children with Attention Deficit Disorder. Society of Biological Psychiatry. 1996; 40: 951-963.
- Chabot RJ, Merkin H, Wood LM, Davenport TL, Serfontein G. Sensitivity and Specificity of QEEG in Children with Attention Deficit or Specific Developmental Learning Disorders. Clin Electroencephalogr. 1996; 27; 26-34.
- Chabot RJ, di Michele F, Prichep L, John ER. The clinical role of computerized EEG in the evaluation and treatment of learning and attention disorders in children and adolescents. J Neuropsychiatry Clinical Neuroscience. 2001; 13: 171-186.
- Dawson G, Warrenburg S, Fuller P. Cerebral laterialization in individuals diagnosed as autistic early childhood. Brain and Language. 1982; 15: 353-368.
- Murias M, Webb SJ, Greenson J, Dawson G. Resting State Cortical Connectivity Reflected in EEG Coherence in Individuals With Autism. Biological Psychiatry. 2007; 62: 270-273.
- Cantor DS, Thatcher RW, Hrybyk M, Kaye H. Computerized EEG analyses of autistic children. J Autism Dev Disorders. 1986; 16: 169-187.
- Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, Elam M, et al. Abnormal EEG lateralization in boys with autism. Clinical Neurophysiology. 2007; 118: 1842-1854.
- Small JH, Small IF, Milstein V, Moore DF. Familial associations with EEG variants in manic-depressive disease. Archives of General Psychiatry. 1975; 32: 43-48.
- Chan AS, Leung W. Differentiating Autistic Children With Quantitative Encephalography: A 3-Month Longitudinal Study. Journal of Child Neurology. 2006; 21: 391-399.
- Rossi PG, Parmeggiani A, Bach V, Santucci M, Visconti P. EEG features and epilepsy in patients with autism. Brain and Development. 1995; 17: 169-174.
- Orekhova E, Stroganova T, Nygren G, Posikera I, Gillberg C, Elam M. P21.5 High frequency activity in ongoing EEG from young children with autism: A two sample study. Clinical Neurophysiology. 2006; 117: 218.
- Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, et al. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. NeuroReport. 2006; 17: 1033-1036.
- Silk T, Vance A, Rinehart N, Egan G, O'Boyle M, Bradshaw JL, et al. Fronto-parietal activation in attention-deficit hyperactivity disorder, combined type: functional magnetic resonance imaging study. The British Journal of Psychiatry. 2005; 187: 282-283.
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-Specific Hypoactivation in Prefrontal and Temporoparietal Brain Regions During Motor Inhibition and Task Switching in Medication-Naive Children and Adolescents With Attention Deficit Hyperactivity Disorder. American Journal of Psychiatry. 2006; 163: 1044-1051.
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, et al. Frontostriatal Connectivity and Its Role in Cognitive Control in Parent-Child Dyads With ADHD. American Journal of Psychiatry. 2007; 164: 1729-1736.
- Tamm L, Menon V, Reiss AL. Parietal Attentional System Aberrations During Target Detection in Adolescents With Attention Deficit Hyperactivity Disorder: Event-Related fMRI Evidence. American Journal of Psychiatry. 2006; 163: 1033-1043.
- Castellanos FX, Kelly C, Milham MP. The restless brain: attention-deficit hyperactivity disorder, resting-state functional connectivity, and intrasubject variability. Canadian Journal of Psychiatry. 2010; 54: 665-672.
- Colla M, Ende G, Alm B, Deuschle M, Heuser I, Kronenberg G. Cognitive MR spectroscopy of anterior cingulate cortex in ADHD: Elevated choline signal correlates with slowed hit reaction times. Journal of Psychiatric Research. 2008; 42: 587-595.
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005; 128: 268-276.
- Waiter GD, Williams JHG, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004; 22: 619-625.
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007; 68: 1718-1725.
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004; 55: 323-326.
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage . 2004; 23: 364-369.
- Hollander E, Soorya L, Wasserman S, Esposito K, Chaplin W, Anagnostou E. Divalproex sodium vs. placebo in the treatment of repetitive behaviors in autism spectrum disorder. International Journal of Neuropsychopharmacology. 2005; 8: 1-5.
- Monastra VJ, Lynn S, Linden M, Lubar JF, Gruzelier J, LaVaque TJ. Electroencephalographic Biofeedback in the Treatment of Attention-Deficit/Hyperactivity Disorder. Applied Psychophysiology and Biofeedback. 2005; 30: 95-114.
- Coben R. Connectivity-guided neurofeedback for autistic spectrum disorder. Biofeedback. 2007; 35: 131-135.
- Cannon R, Congredo M, Lubar J, Hutchens T. Differentiating a network of executive attention: LORETA neurofeedback in anterior cingulate and dorsolateral prefrontal cortices. Int J Neurosci. 2009; 119: 404-441.
- Saletu B, Anderer P, Saletu-Zyhlarz GM. EEG topography and tomography (LORETA) in the classification and evaluation of the pharmacodynamics of psychotrophic drugs. Clinical EEG and Neuroscience. 2006; 37: 66-80.