
Research Article
Austin J Autism & Relat Disabil. 2016; 2(4): 1028.
Particularities of Visual Scanning in Static vs Dynamic Situations for Asperger’s Subjects: New Advance in ASDs
Giuliani F¹* and Armi ND1,2
1Department of Psychiatry at the University Hospital, CHUV, Switzerland
2Institute of Psychology, University of Lausanne, Switzerland
*Corresponding author:Fabienne Giuliani, Community Psychiatry Unit, Department of Psychiatry at the University Hospital, CHUV, Cery site, 1008 Prilly, Switzerland
Received: June 29, 2016; Accepted: August 01, 2016; Published: August 03, 2016
Abstract
Context: Several previous studies have already used eye-tracking technology to demonstrate the particularities of visual scanning in ASD individuals. In this study, in order to compare two situations, a group of Asperger’s individuals and a control group were subject to experimental conditions: a static and a dynamic situation. The goal was to first compare the visual scanning between the two groups and then to compare the visual scanning of the Asperger’s group within each condition. We hypothesized that the visual scanning of the Asperger’s group would be different in the dynamic situation, compared to the norm.
Results: We found a significant difference in visual scanning between the Asperger’s group and the control group in both experimental conditions. These results demonstrate the particularities of visual scanning in individuals with Asperger’s syndrome.
Conclusion: According to our results, individuals diagnosed with an ASD have difficulties when confronted with dynamic stimuli. We were able to demonstrate that Asperger’s individuals use their peripheral vision regardless the kind of stimuli. We were also able to conclude that eye-tracking is an effective aid in screening for ASDs.
Keywords: Asperger’s Syndrome; Eye-tracking; Static stimuli vs dynamic stimuli; Screening; Quantitative data vs qualitative data
Introduction
Asperger’s Syndrome is, according to the current DSM-V (2013) [1], a neuro-developmental disorder classed among the larger denomination of Autism Spectrum Disorders (ASD). ASDs are disorders affecting approximately 1% of the population [2] and affect boys four times more than girls [2,3]. ASDs are characterized by diverse and varied symptoms with respect to communication, behavior, and relationship-based difficulties [4].
In the DSM-IV (2000) [5], Asperger’s Syndrome is its own diagnostic category, and does not include mental impairment. For a more detailed and socio-demographic profile of individuals living with Asperger’s Syndrome, see Giuliani and El Korh (2016) [3]. Note that in this study we reference Asperger’s Syndrome because the participants all received their diagnosis according to the criteria of the DSM-IV, in which Asperger’s is a separate diagnostic and is not included within other autism spectrum disorders. However, the characteristics of Asperger’s syndrome are those of ASD.
Visual exploration is directed at salient elements in the environment [6]. Salience is determined by notice ability (i.e., any form of sensory intensity, e.g. size, brightness, suddenness or an unpredictable movement) or novelty (spatial reorganization of familiar elements) [7].
Visual exploration helps to acquire and process information concerning the environment, particularly spatial information, during a spontaneous learning process [8]. Familiarity develops through a dynamic process, i.e., the lowering of response to a stimulus when repeatedly subjected to it. Habit has traditionally been considered as the most simple and most primitive form of learning [9].
Concerning the duration of fixation, Saint-Aubin, Tremblay and Jalbert (2007) [10] provide evidence that the length of time that the gaze falls on an object in a static image is linked to the probability that the participant remembers the object. This is why it is important to study where the participants fix their gaze, and to consider the time spent on the fixation point. The information thus coded is spatial and temporal.
The duration of fixation varies in function of the situation. As Hooge, Vlaskamp and Over (2007) [11] have shown, if the duration of fixation is too short, the image on the retina will be swept away by the following image, even before the information has been analyzed by the visual system.
Studies which use eye-tracking in order to analyze visual scanning show that there are differences between the general population and those diagnosed with an ASD [12-14]. Eye-tracking is able to measure several parameters including the number and duration of fixation points as well as pupil diameter [15, 16]. Furthermore, it has proven to be a helpful tool for cognitive behavioral therapies when treating these disorders [15-17].
Based on the many studies using eye-tracking and the replication of results from studies conducted with this tool, we can envision the use of oculometry as an effective screening method for ASDs. Essentially, studies show an important contribution of eye-tracking during the processing of stimuli, making it possible to detect disorders [12], as well as an early screening which facilitates the undertaking of rapid treatment [18], and identifying children who are at-risk [19].
From an early age, babies are more attracted by dynamic stimuli than static ones [20], and have a preference for biological or human movements [21,22]. Tardif and Gepner (2009) [20] report that autistic children show differences at the dynamic level, and that the vision of movement proves to be lacking, while their vision of static stimuli is normal compared to children with normal development [19]. Deficits in dynamic vision explain in part the differences of individuals living with an autism spectrum disorder or Asperger’s, like communication and social interactions [23] as well as motor skills [24].
To understand these particularities in individuals with an ASD, the use of eye-tracking is an effective method to understand the visual scanning of static and dynamic stimuli [12]. This is the method that will be used in this study.
Materials and Methods
Sample
A total of 58 adults participated in the study, 24 with an Asperger’s diagnosis (41.37%) and 34 control subjects (58.62%). The sample was comprised of 27 women (46.55%) and 31 men (53.45%). The subjects of the experimental group received a diagnosis according to the criteria of the DSM-IV: (1) a qualitative impairment in social interactions, (2) restricted repetitive and stereotyped patterns of behavior, interests and activities (3) impairment leads to a marked clinically-significant impairment in social, professional and other areas, (4) still at the clinical level, no significant delay in cognitive development during childhood or development, and (5) the disorder cannot fulfill the diagnostic criteria for schizophrenia or another developmental disorder.
Participants with an Asperger’s diagnosis were treated in a department of Psychiatry of Mental Development (SPDM), belonging to the Vaud University Hospital (CHUV). The “control” group was recruited through advertising among the staff of the department, all of which had obtained a secondary level of education. Everyone signed a consent form concerning the use of their clinical data for research purposes. All data was confidential and made anonymous.
Experimental protocol and stimuli
Based on the empirical data views introduction, we came up with a hypothesis that visual scanning of Asperger’s individuals must be different compared to the norm in a dynamic situation. To confirm or refute our hypothesis, we used two types of stimuli: a static one and a dynamic one.
The static stimulus consisted of putting the person in front of a simple matching task on a computer. On the left side of the computer screen was the image to be matched, and on the right side there were four suggestions; the person had to find the matching image from among the four propositions. This task was developed and published by Hadjikhani, Joseph, Snyder, and Tager-Flusberg (2007) [25]. In this “static” situation, we measured the fixation points, their length as well as how the movement of visual scanning was organized.
For the dynamic stimulus, the individuals were asked to take a walk through our facility, following the same path and without receiving any particular instruction. Similar to the static situation, we also measured the fixation points, their length as well as how the movement of visual scanning was organized.
To evaluate the two types of stimuli, eye-tracking (ASL, for more information on the tool, see Giuliani and El Korh (2015) [15] and Giuliani, Favrod, Bonsack, & Schenk (2009) [17]) was used to record the visual scanning of the participants.
Data analysis
Data analysis was done using the Statview 5.0 software. Repeated measures ANOVA were conducted to test the differences between the two groups, between the sexes and the interaction between each group and each sex. Unpaired T-tests made it possible for us to see if there were any differences between the sexes between the types of stimuli and within each group. Also, graphs were created to visualize the fixation point averages as a function of group and sex.
Results
Static situation - Simple matching on a computer
The results of the repeated measures ANOVA on the average of the fixation points made in the simple matching situation indicate a significant different between the groups. On average, the Asperger’s group made significantly more fixation points (μ=.606±.086) compared to the control group (μ=.421±.092 ), with ANOVA F (1,22)=34.266, p<.0001. There was no sex effect (F (1,22)=3.449, p=.0767) nor any interaction effect between the group and sex (F (1,22)=0.264, p=.6124). This significant difference shows that the Asperger’s group has a significant slowing of visual scanning.
The unpaired T-tests on the average duration of the fixation points gave us non significant results, implying that there was no difference between sex and this was for neither the Asperger’s group (t (22)=2.006, p=.0586) nor the control group (t (22)=-.693, p=.4957). This indicates that the average duration of the fixation points, within each group, is fairly the same between men and women. For a more detailed view of these results, see Figure 1.
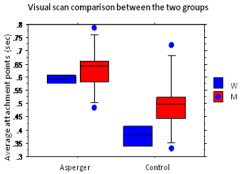
Figure 1: Static situation: comparison of fixation point averages between the
Asperger’s group and the control group as a function of sex. With a scale
going from 0.3 to 0.8 for the averages, the latter are calculated in seconds.
The x-axis indicates the group and the legend the sex.
Dynamic situation - Walk through our facilities
The duration of the fixation points was significantly higher for the Asperger’s group (μ=.345± .099) than for the control group (μ=.264±.049). Indeed, we found a group effect (F(1,17)=9.616, p=.0065), but no interaction effect of the group or of sex (F (1,17)=1.460, p=.2435).
The unpaired T-tests showed that there was no difference between the sexes, in neither the Asperger’s group (t (17)=-1.192, p=.2495) nor the control group (t (17)=-1.185, p=.2.522), indicating that there were no differences between men and women in terms of average fixation points. To see these results, see Figure 2.
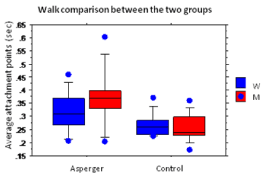
Figure 2: Dynamic situation: comparison of fixation point averages between
the Asperger’s group and the control group as a function of sex. With a scale
going from 0.15 to 0.65 for the averages, the latter are calculated in seconds.
The x-axis indicates the group and the legend the sex.
Quantitative data
Comparison of the two situations (static vs dynamic): We found that the average of the fixation points was larger for both groups in the static situation (simple matching on a computer) than it was in the dynamic situation (the walk). Based on our knowledge [26], we hypothesize that, effectively, in the matching situation the person must process more information in order to carry out the task, which translates to longer fixation points. This is most likely a quantitative difference, meaning a simple difference in the quantity of information to be processed. According to our hypothesis, it is normal that the length of the fixation points in the dynamic situation would be lower, because from a quantitative perspective, there is less information to process.
Asperger’s vs Control comparison: We found a significant difference between the two groups in the dynamic situation. Our results thus confirm the tendency of Asperger’s individuals to make longer fixation points, which is shown by a higher average of those points.
Although the Asperger’s group was capable of matching the static images, there did appear a quantitative difference in the processing of visual information between the two groups, no matter the types of stimuli. Indeed, in this situation, the duration of the fixation points for the Asperger’s individuals was much higher compared to the control group. We based on the work of Hooge et al. (2007) [11] and Land (2009) [27], we hypothesize that Asperger’s individuals use their peripheral vision to process static information, which results in longer fixation points, which is not the case in the control group. On this point, several previous studies for example, Tardif and Gepner (2009) [20] have shown that individuals with Asperger’s Syndrome have a preference for their peripheral vision over their central vision.In other words, the difference of the averages between the groups is not a consequence of the performance of each group, but caused by the way in which surrounding information is processed.
Qualitative data
Our experimental protocol made it possible for us to demonstrate the strategies employed in visual scanning as a function of the task required of an Asperger’s individual. Referring to the work mentioned in the introduction [7-9, 27, 28], we report that the strategies used in the control group differed depending on the task. The strategy used in the static situation was different to the one used in the dynamic situation, as was shown by the difference in the fixation points average. The control group alternated between central vision and peripheral vision depending on the task to be accomplished.
Opposite to this, we report that the Asperger’s group used a single strategy in both conditions. The duration of the fixation points did not differ between the two situations (static and dynamic). In essence, there was no significant difference between the two situations in the Asperger’s group.
Discussion
Looking again at the hypothesis and the results
Again, we postulated that the visual scanning of the Asperger’s group would be different compared to the control group in the dynamic situation. Surprisingly, more information than this was discovered. Essentially, our results indicate a significant difference between the Asperger’s group and the control group in the static situation—the Asperger’s group had much longer fixation points compared to the control group.
We also found this significant difference between the two groups in the dynamic situation, thus confirming our initial hypothesis. This last point demonstrated that the duration of the fixation points was higher in the Asperger’s individuals.
Our results also indicated that the average of the groups between the two types of situations was different. Indeed, the averages were higher in the static situation compared to the dynamic situation, which can be explained by the fact that the quantity of information to be processed was not the same between the static situation and the dynamic situation.
Nevertheless, we found no difference between the men and the women within each group for both situations.
The results of this study highlight a question concerning the single strategy of Asperger’s individuals, which is in line with a reduction in mental flexibility and parallel processing. Very briefly, parallel processing is necessary for optimal executive functioning so that sensory data can be processed effectively. And, according to [15, 29, 30], parallel processing in Asperger’s individuals is impaired, translating into a dominance by one of the brain’s hemispheres, which then explains this lack of sensory data integration. This is also linked to the reduced mental flexibility in Asperger’s individuals, a reduction which leads to an impairment in the integration and management of multiple information [28].
Interpretation of results
According to our results, Asperger’s individuals have significantly longer fixation points, and this is regardless the type of stimuli, what makes us think that individuals with Asperger’s use their peripheral vision to process static and dynamic informations. In comparison, the control group demonstrated a net differentiation between the static situation and the dynamic situation. More precisely, it is true that there were differences between the two situations for the control subjects, but the difference in averages between the static situation and the dynamic situation was not as high as the difference between the two situations for the Asperger’s subjects. Essentially, for the control subjects the simple matching task is a cognitive task in which central vision is favored in order to process the information, while the walk is a procedure and therefore an automatism [30]. For the Asperger’s individuals, a hypothesis is given concerning the fact that peripheral vision is employed for the static stimuli [29,31]. The strategy of using peripheral vision for this type of task is essentially to be expected when the goal is to reduce the flow of information being processed in real time [20,30] and in parallel. This is a method of adapting to their particularities. While the dynamic situation, the walk, is not an automatic procedure like it was for the control subjects. It is difficult to process several elements at one time [32], which is why Asperger’s subjects do not explore a surrounding environment in detail. They are focused on the “fact of walking” and not on the visual area, they do not engage in parallel processing of incoming information. In other terms, walking is processed in a sequential manner in these individuals [30] and not in an overall way.
In terms of the results on the differences between the sexes, there were no significant differences. This lack of agreement may be explained by the small number of subjects in this study.
In conclusion this information suggests eye-tracking can be used for diagnostic procedures for adult.
Limitations and contributions of this study
Our study has three main limitations. First, a larger sample size would enable us to further confirm our data. We had 58 individuals who participated. Also, the situations themselves, which were new experiences for the participants, could lead to some stress. Indeed, several elements may have generated stress, such as the technique, the location and the simple fact of conducting a task with a computer. Nevertheless, both groups were subject to these new experiences. The last limitation is the use of adults. Most diagnoses for ASD and Asperger’s occur when the individuals are children. As such, it is unclear that these data from adults are relevant to diagnostic procedures with children.
This study brings several contributions. First of all, we did not require “performances”, meaning that we did not evaluate the scores. This decreased pressure for the participants because they were not asked to respond correctly to a task. There was no right or wrong in the instructions, only to complete each task as naturally as possible. Finally, we saw that eye-tracking facilitated certain advances in the understanding of these disorders as well as in the manner these individuals process information. To conclude, we can see that eyetracking can help diagnose autism spectrum disorders in terms of understanding the way in which these individuals extract information from social and other situations [19].
Conclusion
Further studies should be conducted with the same experimental protocol but with a larger study sample in order to verify our results. Also to see if there are differences between men and women in control groups in terms of information processing between static and dynamic situations, and to see if these differences might also be found in the Asperger’s population.
But more than anything, to continue to use these experimental protocols using static and dynamic stimuli in order to validate this method for screening for autism spectrum disorders.
References
- DSM-V. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. American Psychiatric Association: Washington, DC. 2013; 1002.
- Hadjikhani N. Scientifically deconstructing some of the myths regarding autism. Swiss archives of neurology and psychiatry. 2014; 165: 272-276.
- Giuliani F and P. El Korh. Adult with Autistic Spectrum Disorders: Specialized Treatment. Advanced Techniques in Biology & Medicine. 2016; 4: 164-168.
- Balfe M and D Tantam. A descriptive social and health profile of a community sample of adults and adolescents with Asperger syndrome. BMC Res Notes. 2010; 3: 300.
- DSM-IV-TR: Diagnostic and statistical manual of mental disorders. 4th edition, text revision edition. American Psychiatric Association: Washington, DC. 2000; XXXVII, 943.
- Berlyne D.E. Conflict, arousal and curiosity. New York: McGraw-Hill. 1960; 350.
- O’Keefe J and L Nadel. The hippocampus as a cognitive map. Oxford: Oxford University Press. 1978.
- Welker WI. An analysis of exploratory and play behavior in animals, in the functions of varied experience, D.W.M. Fiske, S.R., Editor. Homewood: Dorsey. 1961.
- Chapouthier G. Mémoire et cerveau: biologie de l''apprentissage. collection "Science et Découvertes". Paris: Editions du Rocher. 1988.
- Saint-Aubin J, Tremblay S and Jalbert A. Eye movements and serial memory for visual-spatial information: does time spent fixating contribute to recall? Journal of Experimental Psychology. 2007; 54: 264-272.
- Hooge I, Vlaskamp B and E. Over, Saccadic search: on the duration of a fixation, in Eye movements: a window in mind and brain. R. Van Gompel et al. Editors. Elsevier: Oxford. 2007; 582-595.
- Debbané M, Murrey R, Damsa C, Cocchi L , Glaser B, Eliez S. Traitement visuel et cognition sociale chez des enfants et adolescents avec traits autistiques. Neuropsychiatrie de l'enfance et de l'adolescence. 2010; 58: 463-468.
- Johnson BP, Rinehart NJ, White O, Millist L, Fielding J. Saccade adaptation in autism and Asperger's disorder. Neuroscience. 2013; 243: 76-87.
- Joosten A, Girdler S, Albrecht MA, Horlin C, Falkmer M, Leung D, et al. Gaze and visual search strategies of children with Asperger syndrome/high functioning autism viewing a magic trick. Dev Neurorehabil. 2016; 19: 95-102.
- Giuliani F and P El Korh. Case study: the contribution of the neurosciences in the treatment of the anxiety disorders at two people living with autism spectrum disorders and intellectual disabilities. RFCCC, 2015; 10: 8-22.
- Giuliani F, Perrenoud V and Favrod J. Using eye-tracking as support for the TEACCH program and two teenagers with autism-spectrum disorders. Revue Francophone de Clinique Comportementale et Cognitive. 2014; XIX: 39-56.
- Giuliani F, Favrod J, Bonsack C and Schenk F. Organisation de l'exploration oculaire dans le cadre d'un traitement de l'évitement du contact visuel, in TCC et neurosciences. Masson. Editor. 2009.
- Falck-Ytter T. Méthodes innovantes pour l'étude des nourrissons à risque d'autisme. Le Bulletin scientifique de l'arapi. 2014; 34: 14-16.
- Schaer M, Franchini M and Eliez S. Latest findings in autism research: How do they support the importance of early diagnosis and immediate intervention? Swiss archives of neurology and psychiatry. 2014; 8: 277-289.
- Tardif C and Gepner B. Particularités de traitement des informations sensorielles dynamiques chez les personnes présentant des désordres du spectre autistique. Le Bulletin scientifique de l'arapi. 2009; 23: 38-45.
- Bardi L, Regolin L and Simion F. Biological motion preference in humans at birth: role of dynamic and configural properties. Dev Sci. 2011; 14: 353-359.
- Bidet-Ildei C, Kitromilides E, Orliaguet JP, Pavlova M, Gentaz E. Preference for point-light human biological motion in newborns: contribution of translational displacement. Dev Psychol. 2014; 50: 113-120.
- Falck-Ytter T, Von Hofsten C, Gillberg C, Fernell E. Visualization and analysis of eye movement data from children with typical and atypical development. Journal Autism Dev Disord. 2013; 43: 2249-2258.
- Gepner B, Le monde va trop vite pour les personnes autistes ! Hypothèses neurophysio-psychopathogéniques et implications rééducatives. Neuropsyhiatrie de l'Enfance et de l'Adolescence. 2006; 54: 371-374.
- Hadjikhani N, Robert M. Joseph, Josh Snyder and Helen Tager-Flusberg. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007; 28: 441-449.
- Tatler BW. Yarbus, eye movements, and vision. Iperception. 2010; 1: 7-27.
- Land MF. Vision, eye movements, and natural behavior. Vis Neurosci. 2009. 26: 51-62.
- Giuliani F and P El Korh. Psychothérapie de personnes vivant avec le syndrome Asperger autour de la caetextia. Swiss Archives of Neurology and Psychiatry. 2014; 165: 298-305.
- Conil E, Jean-Louis Stilgenbauer, Marie-Christine Mouren, Veronique Gousse. Role de la flexibilité cognitive dans la reconnaissance d'expressions émotionnelles chez les personnes atteintes de Troubles du Spectre Autistique. Annales Médico-Psychologiques. 2014; 172: 392-395.
- Giuliani F and P El Korh. Troubles du spectre de l'autisme: stratégies compensatoires. Swiss Archives of Neurology. Psychiatry and Psychotherapy. 2016; 167: 125-129.
- Planche P. Les modalités du traitement de l'information chez les enfants autistes. Ann Méd Psychol. 2002; 160: 559-564.
- Charrier A, Tardif C and Gepner B. Impact du ralentissement des informations faciales dynamiques dans l'autisme: une étude en oculométrie. le Bulletin scientifique de l'arapi. 2014; 33: 33-38.