
Editorial
J Bacteriol Mycol. 2014;1(1): 2.
Update on Vaginal Lactobacilli and Biofilm Formation
Gary Ventolini*
Department of Obstetrics and Gynecology, Texas Tech University Health Sciences Center, USA
*Corresponding author: Gary Ventolini, Department of Obstetrics and Gynecology, Texas Tech University Health Sciences Center, USA.
Received: July 11, 2014; Accepted: July 15, 2014; Published: July 26, 2014
Albert S Döderlein (a German physician) described in 1892 an organism that was isolated from a vaginal specimen of asymptomatic pregnant women. He named it Doderlein’s bacillus and was later renamed Lactobacillus [1]. Döderlein further considered and decided to divide the bacterial groups that he founded into normal (Grade A: dominated by the vaginal Döderlein’s bacillus) and abnormal (Grade C: dominated by other micro-organisms). He also argued that in the normal vaginal secretions, the combination of Döderlein’s bacilli and acidity were essential to keeping the vagina free of pathogenic bacteria [1]. This concept was fundamental and still continues to be utilized by contemporary vaginal investigators.
Over the past 4 or 5 years the advent of competing technologies, high-throughput sequencing (Pyrosequencing), software for computational analysis and genus-specific quantitative PCR (qPCR) assays have validated significant observations made by Döderlein and contemporaries(the protective role of lactobacilli and the importance of endogenous and exogenous microbes in the pathogenesis of diseases) [2-5]. It has also helped us to identify and characterize the structure of each bacteria and to compare it to the micro-flora of other individuals. Today it is well established that the majority of vaginas from women of child bearing age are colonized by mainly 4 groups of lactobacilli: L. crispatus, L. iners, L. jensenii, and L. gasseri (this is not true across all racial, locations and ethnic groups) [6,7]. Additionally we know that the healthier vaginas harbors mostly L. crispatus and L. jenseniias recently reported by Ravel et al based on pyrosequencing analysis [8].
Human vaginal lactobacilli express vaginotropism and are mainly derived from the gut micro-flora that colonizes the vaginal nutrientrich environment maintaining a stable vaginal flora [9]. The estrogen dependent stratified vaginal epithelium, rich in glycogen, is controlled by the physiologic ovarian activity. The endogenous degradation of glycogen makes glucose accessible. Vaginal lactobacilli are considered to be obligate homo-fermenters of glucose having as end product: lactic acid. Lactobacilli also produce hydrogen peroxide and with lactic acid they are responsible to maintaining vaginal pH between 3.8 and 4.2. Moreover lactobacilli also produce hydroxyl radicals and bacteriocins (small peptides with microbicide activity) which help in keeping other bacteria under control [10,11]. Other recognized lactobacilli secretions include organic acids, bio-surfactants, and arginine deaminizes that allowed specific receptors to adhere to the vaginal epithelium and co-aggregate to form biofilms [12].
Almost all lactobacilli have a small genome of approximately 1.8– 2 Mb, due to a number of evolutionary adaptations including losses of genes for biochemical activities as well as acquirements of essential genes by horizontal transfer through bacterial conjugation and phage infection.12Such new genes have contributed to “protein transporter systems” that facilitates lactobacilli vaginal colonization. Mendes- Soares et al. found that the genomes of lactobacilli species that exhibit vaginotropism were significantly smaller and had significantly lower GC content than those of the nonvaginal species [13]. The genes related to the bacteriocin production were recently identified and sequenced by Stoyancheva et al. [14].
Biofilm Formation by Lactobacilli
Bacteria frequently live as complex conglomerates known as biofilms. In general, single-species biofilm formation involves two main independent steps: initial adhesion to the surface and biofilm accumulation [15].
Biofilm formation is a sophisticated process that includes the recognition of surface-related stimuli to enable adhesion and a matrix production that is extraordinarily complex in its structure and function. This matrix is identified as the extracellular polymeric substances (EPS) and within it bacteria are intertwined in.
Recently we have learned about the contribution of genetic determinants in biofilms formation and how the environmental conditions influence the process. Additionally it is now known that bacteria exhibit biofilm-linked traits and heightened tolerance to antibiotic treatment and host defenses [16].
Lately lactobacilli were studied by Gomaa for biofilm formation in different growth media. All lactobacilli isolate produced biofilm on polystyrene surface. L. acidophilus showed the highest biofilm formation. He reported auto and co-aggregation with three pathogenic bacterial strains [17].
Last year, Ventolini verbally presented at COGI in Vienna findings regarding biofilms produced by human vaginal lactobacilli (manuscript under consideration by Medical Hypothesis) [18]. Follow-up qPCR research categorized these lactobacilli as L. jensenii (to be presented at COGI in Paris). A microscopic photograph (normal saline vaginal wet mount, 400 x magnifications) is included as an early release Figure 1.
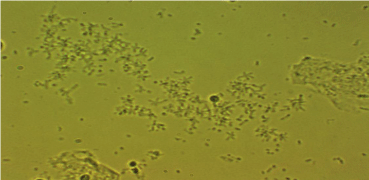
Figure 1: Biofilm production by Lactobacilli Jensenii in human vagina (normal saline mount).
Conclusion
Lactobacilli are responsible for maintaining the natural healthy micro-flora balance in the vagina. Our knowledge has been progressive and we have now a more complete interpretation of the intricacies and interactions between the diverse vaginal micro-flora and lactobacilli. Clinical research is still necessary to identify the contributions that lactobacilli biofilm could make regarding preterm labor prevention and protection against recurrent bacterial and fungal infections.
References
- Hillier S, Marrazzo J, Holmes KK. Bacterial Vaginosis. Holmes KK, Sparling FP, Stamm, WA. Sexually transmitted diseases. 4th edn. McGraw Hill Medical. 2008; 737-768.
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009; 75: 7537-7541.
- Lozupone C, Hamady M, Knight R. UniFrac--an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 2006; 7: 371.
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; 7: 335-336.
- Zozaya-Hinchliffe M, Lillis R, Martin DH, Ferris MJ. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J Clin Microbiol. 2010; 48: 1812-1819.
- Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007; 1: 121-133.
- Nam H, Whang K, Lee Y. Analysis of vaginal lactic acid producing bacteria in healthy women. J Microbiol. 2007; 45: 515-520.
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011; 108 Suppl 1: 4680-4687.
- Hyman RW, Fukushima M, Diamond L, Kumm J, Giudice LC, Davis RW, et al. Microbes on the human vaginal epithelium. Proc Natl Acad Sci U S A. 2005; 102: 7952-7957.
- Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996; 174: 1058-1063.
- Aroutcheva AA, Simoes JA, Faro S. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect Dis Obstet Gynecol. 2001; 9: 33-39.
- Lepargneur JP, Rousseau V. [Protective role of the DoderleÏn flora]. J Gynecol Obstet Biol Reprod (Paris). 2002; 31: 485-494.
- Mendes-Soares H, Suzuki H, Hickey RJ, Forney LJ. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J Bacteriol. 2014; 196: 1458-1470.
- Stoyancheva G, Marzotto M, Dellaglio F, Torriani S Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch Microbiol. 2014.
- Cerca N, Pier GB, Vilanova M, Oliveira R, Azeredo J. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res Microbiol. 2005; 156: 506-514.
- Wozniak DJ, Parsek MR. Surface-associated microbes continue to surprise us in their sophisticated strategies for assembling biofilm communities. F1000Prime Rep. 2014; 6: 26.
- Zakaria Gomaa E. Antimicrobial and anti-adhesive properties of biosurfactant produced by lactobacilli isolates, biofilm formation and aggregation ability. J Gen Appl Microbiol. 2013; 59: 425-436.
- Ventolini G. Biofilm formation by vaginal lactobacillus in vivo. COGI Breakthrough & Innovation Session. October 25, 2013.