
Research Article
J Bacteriol Mycol. 2023; 10(2): 1205.
Candidemia - Species Distribution and Antifungal Resistance Patterns in Two Tertiary Care Centers in South India
Nimmala P¹*; Prakasham S²; Anand M¹
1Department of Clinical Microbiology, Yashoda Hospital Secunderabad, India
2Department of Clinical Microbiology, Yashoda Hospital Somajiguda, India
*Corresponding author: Pavani Nimmala Department of Clinical Microbiology, Yashoda Hospital, S.P Road, Secunderabad, 50000, Telangana, India. Tel: 919959388055 Email: drpavani12@gmail.com
Received: April 04, 2023 Accepted: May 16, 2023 Published: May 23, 2023
Abstract
Background: Bloodstream infections due to Candida species are a significant cause of morbidity and mortality in hospitalized patients worldwide. Epidemiology of Candidemia is dynamic with reports of non-albicans Candida species on a steady increase and the emergence of C.auris reported from many centers in India. This study was undertaken to analyze species distribution and antifungal susceptibility of Candida bloodstream infections in two tertiary care centers in Telangana, South India.
Material and Methods: In a retrospective analysis, Candida isolates from blood cultures of patients with suspected bloodstream infection over five years (2017-2021) were identified and tested for antifungal susceptibility by Vitek 2 Compact (Biomerieux, France).
Results: A total of 85,603 blood cultures were processed from two centers from January 2017 to December 2021. Among these, 270 (0.31%) Candida species were isolated. Of the total Candida isolates, C. tropicalis (49.2%) was the most common isolate, followed by C. albicans 55 (20.3%), C. glabrata 35 (12.9%), C. parapsilosis 25 (9.2%), C. auris (5.5%), C. guilliermondii (1.4%) and C. krusei (1.1%). We have tested six antifungals and of the total isolates, resistance to fluconazole was highest (11.9%) followed by amphotericin B (7.0%), caspofungin (2.9%), voriconazole (2.3%), flucytosine (1.8%) and micafungin (0.7%). C.auris isolates were found to be resistant to two or three classes of antifungals tested and 13.3% were pandrug -resistant.
Conclusion: C.tropicalis was the most common isolate and each Candida species showed varied antifungal resistance rates. We emphasize the need for the identification of Candida species and antifungal susceptibility for every invasive isolate to tailor targeted preventive and therapeutic antifungal strategies in line with current evidence and guidelines.
Keywords: Candidemia; Candida species; Antifungal resistance
Introduction
Over the past decade, there has been a steady increase in the incidence of Invasive Fungal Infections (IFI), particularly candidemia. As a result of advances in medical care, there are more immunocompromised patients as well as critically ill patients who are prone to fungal infections in the hospital setting. Exposure to broad-spectrum antibiotics, the Central Venous Catheter (CVC), recent major surgery, necrotizing pancreatitis, total parenteral nutrition, and diabetes mellitus are common risk factors for candidemia [1].
Despite the availability of safe and effective choices of antifungal treatment, candidemia has an overall crude mortality rate of 40-60% [2,3], and early suspicion and prompt treatment with antifungals are critical to enhancing survival. There is considerable geographic variation in the range of Candida species isolated from hospitals. Non-albicans Candida. Has been almost universally prevalent over the last decade, accounting for more than 50% of all Candida isolates [3]. With the growing resistance to commonly used antifungals and the emergence of multidrug-resistant C.auris, it is essential to know the local epidemiology of Candida species and rates of antifungal resistance to make informed therapeutic decisions while awaiting culture and susceptibility data.
Data regarding the epidemiology of Candida is scarce from the south India. This study aims to describe the epidemiology of Candida species isolated from two tertiary care hospitals in South India over 5 years from January 2017 to December 2021 and compare the findings with other published studies from India. To our knowledge, this is the only study from South India reflecting data collected over more than 2 years duration.
Materials and Methods
A retrospective observational study was conducted in the Department of Clinical Microbiology in two tertiary care centers in Hyderabad, Telangana. All patients with suspected bloodstream infections admitted to the hospital from January 2017 to December 2021 were included in the study. Candidemia was defined as the isolation of Candida species from blood cultures in a patient with signs and symptoms of bloodstream infections. Demographic data of all patients such as age, gender, and location were derived from the hospital medical records system.Candida species were isolated from blood samples using BacT/Alert ((Biomereiux, France), and identification with antifungal susceptibility testing was done by Vitek 2 Compact (Biomereiux, France) using ID-YST and AST-YS08 cards respectively according to manufacturer's instructions. A total of six antifungals were tested including amphotericin B, fluconazole, voriconazole, caspofungin, micafungin, and flucytosine. For Candida species except for C.auris, MIC breakpoints were interpreted as per CLSI M27-A3 supplement for yeasts [4]. For C. auris breakpoints were defined based on expert opinion as released by the US Centers for Disease Control and Prevention (CDC) in October 2017 and modified in April 2019 [5,6]. Microsoft Excel Ver 16.16.17 (2018) was used to tabulate and statistically analyze the results.
Results
A total of 85,603 blood samples from patients with suspected bloodstream infection were processed during the five years study period (January 2017 to December 2022) with a prevalence of 0.31% candidemia. The age of the patients with candidemia ranged from 21 days to 80 years with a median age of 58 years among them 210(77.7%) were males and 60(22.2%) were females, of which 192(71.1%) were from ICU and 78(28.8%) were from wards. Among Candida species, C. tropicalis 133(49.2%) was the commonest isolate, followed by C. albicans 55(20.3%), C.glabrata 35(12.9%), C. parapsilosis 25(9.2%), C.auris 15(5.5%), C. guilliermondii 4(1.4%) and C. krusei 3(1.1%) (Figure 1). Of the total candida isolates resistance to fluconazole was highest (11.9%) followed by amphotericin B (7.0%), caspofungin (2.9%), voriconazole (2.3%) and (1.5%) flucytosine (1.8%) micafungin (0.7%) (Table 1). Among seven species of candida isolated, C.auris were (100%) resistant to amphotericin B and fluconazole, (23%) to flucytosine, (13.3%) to caspofungin, (13.3%) to micafungin. Of the total 15, C.auris isolate 2 (13.3%) were pandrug- resistant.
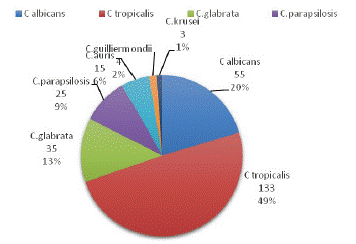
Figure 1: Species distribution of Candida isolates.
Candida species
Amphotericin B n(%)
Fluconazole n(%)
Voriconazole n(%)
Caspofungin n(%)
Micafungin n(%)
Flucytosine n(%)
C tropicalis (n=133)
4(30.0%)
8(6.0%)
5(3.7%)
0(0%)
0(0%)
2(1.5%)
C albicans (n=55)
0(0%)
1(1.8%)
1(1.8%)
0(0%)
0(0%)
0(0%)
C.glabrata (n=35)
0(0%)
0(0%)
0(0%)
6(17.1%)
0(0%)
0(0%)
C.parapsilosis (n=25)
0(0%)
7(28%)
0(0%)
0(0%)
0(0%)
0(0%)
C.auris (n=15)
15(100%)
15(100%)
0(0%)
2(13.3%)
2(13.3%)
3(20.0%)
C.guilliermondii (n=4)
0(0%)
1(25%)
0(0%)
0(0%)
0(0%)
0(0%)
C.krusei (n=3)
0(0%)
*na
0(0%)
0(0%)
0(0%)
0(0%)
Total (n=270)
19(7.0%)
32(11.9%)
6(2.3%)
8(2.9%)
2(0.7%)
5(1.8%)
Table 1: Antifungal resistance of each Candida species isolated.
Discussion
Candidemia is an emerging problem in healthcare settings worldwide. In the present study, the incidence of Candida bloodstream infection was 0.31%. A great variation in the incidence of candidemia has been reported in India with isolation rates ranging from 0.65% to as high as 18.86% has been reported in various studies from the subcontinent [7-15]. It is interesting to note that most of these studies are from the northern part of the country and the occasional study we found reported from South India had lower isolation rates of Candidemia (0.65%, 1.05%) similar to our study [14,15]. In a nationwide, multicentric study performed at 27 Indian ICUs, Chakrabarti et al. reported an overall incidence of 6.51 cases per 1,000 ICU admissions [16].
The distribution of Candida species isolated from blood varies markedly between different geographical areas in the world. Studies from India's tertiary care centers have reported a steady rise in the incidence of candidemia caused by non-albicans Candida, with isolation rates varying from 50 to 96% [17]. In our study also, non-albicans Candida (79.6%) bloodstream infections were more common. This finding is consistent with other studies not only from the Indian subcontinent but also from Asia, Southern Europe, and South America [18]. In contrast, C. albicans is still reported to be the most common isolate in Northern Europe and the USA [19].
More than 90% of invasive infections are attributed to five species: C. tropicalis, C. albicans, C. glabrata, C. parapsilosis, C. guilliermondii, and C. Krusei [20]. In our study also, these five species were predominantly isolated and in addition, we isolated C auris accounting for 5.5% of the total 270 isolates. Similar to our study, in India, C. tropicalis ranks first as a cause of candidemia, reported in as many as 34.4-74.6% of cases [7,10-11,16,17,21,22]. These studies also underline the declining incidence of candidemia caused by C. albicans. Also, in line with other studies [11,23-25], C. glabrata was the third most isolated Candida species isolated at our center. The increased isolation of C. glabrata is believed to be a consequence of the increased use of fluconazole within the hospital set-up, especially as prophylaxis for immunosuppressed patients or patients of suspected sepsis with multiple invasive intravascular catheters. Due to the same reason, there has also been an increase in the incidence of C.krusei. Fluuconazole MICs are naturally high in C.glabrata isolates and C.krusei is considered to be intrinsically resistant to fluconazole [26].
C.parapsilosis isolation has been associated with vascular catheters, prosthetic devices, and parenteral nutrition [27] and is an emerging cause of candidemia in India. In our study, we have isolated 9.2 % of C.parapsilosis similar to other studies from India [16,21,28]. Earlier, C. guilliermondii was thought to be an animal saprophyte with little or no involvement in human infection. Over the recent past, there is a rise in the infections caused by C. guilliermondii, with reported incidence ranging from 0.6 % in North America to 3.7 % in Latin America [29] and 0.95-10.1% from India [7,22,28] and there was an overall decline in isolation of C. guilliermondii and C.krusei during the study period.
C.auris at our institute was first isolated in 2017, confirmation of species identification was done at the Post Graduate Institute of Medical Education and Research, Chandigarh which is the Center for Advanced Research in Medical Mycology in India, and subsequent identification was done by Vitek 2 compact version 8.01. C.auris ranked 5th most predominant isolate causing candidemia at our institute and over the recent 5 years, 2017-2021, we isolated 15 C.auris. Other centers from India have also reported the recent isolation of C. auris indicating that this species is an emerging pathogen in this part of the world [8,22].
A range of antifungal drugs is available belonging to three major classes -polyenes, azoles, and echinocandins. Knowledge of local epidemiology is crucial to decide on the choice of empirical antifungal treatment along with patient co-morbidities and cost of treatment. The emergence of non-albicans Candida and the prevalence of antifungal-resistant strains mandates the identification of all invasive candida species along with its antifungal susceptibility testing for tailoring treatment.
Among the total 270, Candida isolates 11.9% were resistant to fluconazole, and 2.3% were resistant to voriconazole. Fluconazole resistance was not reported for C.krusei in our study as it is considered to be intrinsically resistant and C.glabrata isolates all of which are considered to have intermediate susceptibility to fluconazole [26]. The prevalence of azole resistance from other studies from India varied from 3.3% to 64% [7,13,17,22] for fluconazole and 2.4 to 8.82% for voriconazole [7,1].
The high bioavailability, the multitude of dosage forms, minimal side effects and the relatively low cost make fluconazole preferable antifungal for all types of yeast infections but, there is a growing incidence of its resistance. Further analysis of our study showed that all 15 isolates of C.auris (100%), 7(28%) C.parapsilosis, 1(25%) of C. guilliermondii, and 8(6%) of C.tropicalis isolates were resistant to fluconazole which were the major contributors and C.albicans, showed very low resistance of 1.8%.
In C.auris, a very high resistance to fluconazole of 90-100% has been reported in other studies from India [30,31]. Some authors have suggested that there might be a mechanism of intrinsic resistance to explain such high MICs of >32μg/ml [31-33]. However, in the landmark multicentre study by Chakraborti et al in 2014, [16] the resistance of fluconazole reported against C.auris strains was only 30.8%. A nationwide multicentre study is required to ascertain whether the high resistance is a recent phenomenon and the mechanisms contributing to the development of resistance.
There appears to be a dichotomy in reported resistance rates to fluconazole concerning C. parapsilosis, while Kaur et al [7] have reported low resistance (<10%) over the past 20 years, other studies have reported high resistance of 37.5% [34], 38.5% [21] like our findings of 28%. In a large, international antifungal surveillance program [35] the percentage of resistance is low (<10%) however, the authors have noticed resistance to fluconazole raised eventually in this isolate. Importantly, in our present study, no resistance to azoles among C.glabrata was observed which is similar to one Indian study [36] and other multi-centric studies from Peru [37], indicating the possibility of genetic strain variations in Indian C. glabrata isolates compared to other countries. Very few studies from India have reported resistance to echinocandins. In our study, we have reported resistance to caspofungin in C. glabrata and resistance to both caspofungin and micafungin in C. auris while other species did not show resistance to echinocandins. Other studies from India have reported echinocandin resistance [7,21,34] with higher resistance reported in C. auris, C. glabrata, and C. krusei. Resistance to amphotericin B in our study was also present in all 15(100%) C. auris isolates. This may be explained in part by recent studies which demonstrate that under stress conditions, a hypermutable state may lead to rapid acquisition of resistance to fluconazole, echinocandin, and amphotericin B [35] and it may be why, even though the drug targets and resistance mechanisms are vastly different, resistance too many antifungals develops after exposure to fluconazole.
Our study has certain limitations. It was a retrospective study conducted at two tertiary care hospitals. As such it reflects the epidemiology and antifungal practices within the patient population of our hospital only. Secondly, the antifungal susceptibility test was done by automated colorimetry. Confirmation of resistant strains was not done by a micro broth dilution method. However, in all cases, adequate precautions were taken to ensure that a pure isolate was processed as per the manufacturer’s instructions.
Conclusion
C. tropicalis was the most common isolate causing candidemia. Fluconazole resistance was high with each Candida species showing different antifungal resistance rates. As there is a huge geographical variation in the distribution of candida species and their resistance patterns, it is important to monitor them from time to time. This will not only aid the clinician in the timely institution of directed therapy in the form of appropriate antifungal agents but also contribute to antifungal stewardship.
References
- Pinto-Magalha˜es S, Martins A, Lacerda S, Philipe R, Prista-Leao B, et al. Candidemia in a Portuguese tertiary care hospital: Analysis of a 2-year period. Journal Mycol Med. 2019; 29: 320-324.
- Poissy J, Damonti L, Bignon A, Khanna N, von Kietzell M, Boggian K, et al. Risk factors for candidemia: a prospective matched case-control study. Crit Care. 2020; 24:109.
- Pappas PG, Kauffman KA, Andes DR, Clancy CJ, Marr KA, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2016; 62: e1–e50.
- CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. CLSI document M27–A3, 3rd edn. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI). 2008.
- National Center for Emerging and Zoonotic Infectious Diseases. Algorithm toidentify Candida auris based on phenotypic laboratory method and initialspecies identification. Atlanta, GA: CDC; 2019. 2018.
- US Centers for Disease Control and Prevention (CDC). Candida aurisIdentifi-cation of. Atlanta, GA: CDC; 2018.
- Kaur H, Singh S, Rudramurthy SM, Ghosh AK, Jayashree M, et al. Candidaemia in a tertiary care centre of developing country: Monitoring possible change in spectrum of agents and antifungal susceptibility. Indian J Med Microbiol. 2020; 38: 109-15.
- Nazir A, Masoodi T. Spectrum of candida species isolated from neonates admitted in an Intensive Care Unit of teaching hospital of Kashmir, North India. J Lab Physicians. 2018; 10: 255-259.
- Gandham NR, Vyawahare CR, Jadhav SV, Misra RN. Candidemia: Speciation and Antifungal susceptibility testing from a Tertiary Care Hospital in Maharashtra, India. Med J DY Patil Univ. 2016; 9: 596-9.
- Chaudhary U, Goel S, Mittal S. Changing trends of Candidemia and antifungal susceptibility pattern in a tertiary health care centre. Infect Disord Drug Targets. 2015; 15: 171-6.
- Chander J, Singla N, Sidhu SK, Gombar S. Epidemiology of Candida bloodstream infections: experience of a tertiary care centre in North India. J Infect Dev Ctries. 2013; 7: 670-5.
- Oberoi JK, Wattal C, Goel N, Raveendran R, Datta S, et al. Non-albicans Candida species in bloodstream infections in a tertiary care hospital at New Delhi, India. Indian J Med Res. 2012; 136: 997-1003.
- Kothari A, Sagar V. Epidemiology of candida bloodstream infections in a tertiary care institute in India. Indian J Med Microbiol. 2009; 27: 171-2.
- Giri S, Kindo AJ, Kalyani J. Candidemia in intensive care unit patients: a one year study from a tertiary care center in South India. J Postgrad Med. 2013; 59: 190-5.
- Alagiri SB, Vijayaraman RS, Ramaraj V, Kindo AJ. Invasive yeast infections in the intensive care unit of a tertiary care centre in South India. J Acad Clin Microbiol. 2017; 19: 19-26.
- Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015; 41: 285-95.
- Tak V, Mathur P, Varghese P, Gunjiyal J, Xess I, et al. The epidemiological profile of candidemia at an Indian trauma care center. J Lab Physicians. 2014; 6: 96-101.
- Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: A systematic review. Int J Infect Dis. 2010; 14: e954-66.4.
- Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014; 20: 5–10.
- Giri S, Kindo AJ. A review of Candida species causing bloodstream infection. Indian J Med Microbiol. 2012; 30: 270-8.
- Mathur P, Hasan F, Singh PK, Malhotra R, Walia K, et al. Five-year profile of candidemia at an Indian trauma centre: High rates of Candida auris bloodstream infections. Mycoses. 2018; 61: 674-680.
- Thomas M, Oberoi A, Dewan E. Species distribution and antifungal susceptibility of candidemia at a multispecialty center in North India. CHRISMED J Health Res. 2016; 3: 33-6.
- Gupta A, Varma A, Gupta A. Candida glabrata candidemia: An emerging threat in critically ill patients. Indian J Crit Care Med. 2015; 19: 151-154.
- Kaur R, Jaggi S, Dhakad MS, Rawat D. An etiological and antifungal profile of candidemia in children. Int J Community Med Public Health. 2019; 6: 3899-3904.
- Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014; 20: 5–10.
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed. CLSI Supplement M60. Wayne, PA: Clinical and Laboratory Standards Institute. 2017.
- Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008; 21: 606-625.
- Deorukhkar SC, Roushani S, Bhalerao D. Candidemia due to Non-Albicans Candida Species: Risk Factors, Species Distribution and Antifungal Susceptibility Profile. J Microb Path. 2017; 1: 101.
- Ahangarkani F, Badali H, Rezai MS, Shokohi T, Abtahian Z, et al. Candidemia due to Candida guilliermondii in an immuno-compromised infant: a case report and review of literature. Curr Med Mycol. 2019; 5: 32-36.
- Prayag PS, Panchakshari S, Prayag A. The Dominance of Candida auris: A Single-center Experience of 79 Episodes of Candidemia from Western India. Indian J Crit Care Med. 2022; 26: 558-561.
- Chowdhary A, Sharma C, Meis JF. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017; 13: e1006290.
- Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, et al. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol. 2011; 49: 3139–42.
- Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash A, et al. New clonal strain of Candida auris, Delhi, India. Emerg Infect Dis. 2013; 19: 1670–73.
- Wattal C, Raveendran R, Goel N, Oberoi JK, Rao BK. Ecology of blood stream infection and antibiotic resistance in intensive care unit at a tertiary care hospital in North India. Brazilian Journal of Infectious Diseases. 2014; 18: 245-251.
- Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infectious Diseases. 2019; 6: S79–S94.
- Singh A, Healey KR, Yadav P, Upadhyaya G, Sachdeva N, et al. Absence of azole or echinocandin resistance in Candida glabrata Isolates in India despite background prevalence of strains with defects in the DNA mismatch repair pathway. Antimicrob Agents Chemother. 2018; 62: e00195-18.
- Rodriguez L, Bustamante B, Huaroto L, Agurto C, Illescas R, et al. A multi-centric study of Candida bloodstream infection in Lima-Callao, Peru: species distribution, antifungal resistance and clinical outcomes. PLoS One. 2017; 12: e0175172.