
Research Article
J Bacteriol Mycol. 2024; 11(1): 1214.
First Insight in Fungi Diversity and Mycotoxins Contaminating Smoked Fish Sold in Yaoundé Retails Markets in Cameroon
Olivier Ziem2,3; Gwladys Ekwe Priso1; Amandine Plidikoua1; Moise Ntah A Ayong1; Balbine Adande1; Modeste Lambert Sameza1; Francois-Xavier Etoa3; Francioli Koro Koro1*
¹Department of Biochemistry, Faculty of Sciences, University of Douala, Cameroon
²Department of Animal Biology and Physiology, Faculty of Sciences, University of Douala, Cameroon
³Department of Microbiology, Faculty of Sciences, University of Yaounde I, Cameroon
*Corresponding author: Franciolo Koro Koro Department of Biochemistry, Faculty of Sciences, University of Douala, P.O. Box 12 574, Douala, Cameroon Email: korokorogozion@yahoo.fr
Received: January 10, 2024 Accepted: February 20, 2024 Published: February 27, 2024
Abstract
Mycotoxins are one of the major food poisons for the human’s liver. Their production is generally related to improper food storage condition, mainly cereals and groundnuts. In the present study we assessed the moulds diversity and mycotoxin contamination of smoked fish, one of the most eaten foodstuffs in Cameroon. For this purpose, 1000 specimens of smoked fish were randomly collected from 10 retail markets in Yaoundé and pooled into 50 composite samples. Moulds were isolated by dilution, suspension and culture methods. Identification of fungal was assessed by phonotypical characterization. Three different mycotoxins (Aflatoxin B1, Ochratoxin A and fumonisin B) were tested on fish. Semi-quantitative immuno-chromatographic assay was used for (AFB1) and Fumonisin B (FB), ELISA assay was used for Ochratoxin A (OTA). The identification of moulds flora associated with smoked fish revealed that they belonged to three genuses namely Aspergillus, Penicillium and Absidia. Only species belonguing to Aspergillus genus appear to produce Aflatoxins. Three types of mycotoxin were detected (AFB1, OTA and FB) with occurences of 76%, 18% and 6% respectively at levels above the reference maximum admissived limits.
Keywords: Aspergillus; Fungi isolates diversity; Smoked fish contamination; Mycotoxins; Retails markets; Cameroon
Abbreviations: AFB1: Aflatoxin B1; FB: Fumonisin B; OTA: Ochratoxin A; ISO: International Standardization Organization; rpm: rounds per minute; PDA: Potato Dextrose Agar; OGA: Oxytetracyclin Glucose Agar; NF: Normes Francaises; V/V: volume/volume; CEA: Coconut Extract Agar; UV: Ultraviolet; ppb: part per billion; ELISA: Enzyme-Linked Immunosorbent Assay; HPLC-SM: High Performance Liquid Chromatography couple with Mass Spectrometry
Introduction
Fish in all their forms, remain one of the most used foods in the world, with an average consumption level of 20.1 kg per capita [1]. In Cameroon, fish products contribute up to 25.5% of protein diet of the population [2]. In African countries including Cameroon, smoking fish remains the most suitable method for fish preservation. However, several studies have revealed the presence of pathogenic bacteria such as Staphylococcus aureus, Salmonella spp., Escherichia coli pathotypes, Listeria monocytogenes in smoked fish [3-8] and fungi which can produce mycotoxins under certain conditions such as high temperature and humidity rate [9]. Tropical climate and poor crop storage conditions are frequently responsible of fungal growth and mycotoxin production [10]. Mycotoxins are chemical compound mainly produced by fungi belonging to the Aspergillus, Penicilliums and Fusariums genus [11] which have negative impact on human and animal health [12]. Consumption of mycotoxin contaminated food may induce acute or chronic affections, including non-communicable diseases [13]. According to International Agency for Research on Cancer chronic exposure to Aflatoxin B1 (AFB1) or its precursors has been associated with genotoxicity and hepatocellular carcinoma [14]. Concerning Fumonisin B (FB), studies revealed it association with oesophageal cancer incidence in South Africa and China [15,16]. Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius are the most known species and have been subject of several research studies that have demonstrated their aflatoxin production capabilities [17-20]. In addition to these three species, aflatoxin production capabilities have been discovered more recently in the following Aspergillus species: Aspergillus tamarii, Aspergillus ochraceoroseus [21], Aspergillus pseudotamarii [17], Aspergillus bombycis [22] and Aspergillus rambelli [23]. The occurrence of mycotoxins in Cameroonian food commodities such as maize, peanuts, beans and soybeans has been reported by many authors [24-26]. But there is a lack of data about fungal and mycotoxin contamination of smoked fish. Given the economic and nutrition importance of smoked fish and the increase of gastrointestinal cancers in Cameroonian population, the present study aims to dose mycotoxin and identify fungi contamining and producing aflatoxin on smoked fish sold and intended to human consumption in Cameroon.
Material and Method
Sample Collection
A total of 1000 specimens of smoked fish were randomly collected from ten markets (Figure 1) of Yaoundé urban council during a 6 months period. The different market has been chosed by their capacities of selling many types of smoked fish consumed in Cameroun. Sampling was performed according to ISO 2859-1 Standards [27]. Smoked fish were collected using individuals steriles plastics bags and transported to the laboratory under refrigerated boxes. The specimens were then pooled into 50 composite samples and grouped by species and by markets. All composite samples were grounded and divided into 3 aliquots. One aliquot for moulds isolations, the second aliquot for mycotoxins detection and the third aliquot for humidity rate assessment.
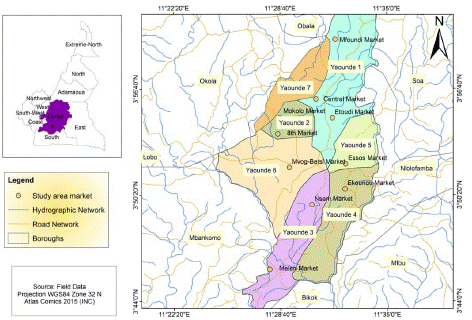
Figure 1: Location map of different markets and their smoked fish collection sites in Yaoundé.
Humidity Rate Assessment
Humidity rate of composite smoked fish samples was measured using a numeric thermohygrometer (traceable reference 620-2273) following manufacturer’s instruction.
Mould Isolation and Identification
Ten grams of each composite sample were added to 90 ml of sterile Buffered Pepton Water (Biokar) and homogenized during 1min using a stomacher (LB 400 VWR) to obtain a 1/10 dilution. The futhers decimals dilutions were prepared in BPW as described by ISO 6887-1: 2017 [28] and inoculated in Potato Dextrose Agar (PDA Biokar) medium and Oxytetracyclin Glucose Agar (OGA Biokar) medium according to NF V 08-59: 2002 standard [29]. All plates were incubated for 5 days at 25°C ± 1°C. After the incubation period molds culture were isolated in OGA and PDA plates for identification assays. The identification of fungal isolates was performed according to standard taxonomic systems based on morphological observation of mycelium, thallus and spore using the dichotomous keys [30]. The microscopy observation with 100x magnification after coloration with lactophenol blue based on the shape of conidiophores and conidia dimension completed the identification algorithm.
Assessment of the Capacity of Fungal Isolates to Produce Aflatoxin
The ability of fungal isolates to produce aflatoxins was performed according to Pane B. Ouattara-Sourabie [31]. Fungal isolates were plated on Coconut Extract Agar (CEA) and incubated at 25°C ±1°C during 7 days. After incubation period, fluorescence under Ultraviolet (UV) radiation was assessed using wood lamp at wavelength of 365 nm. The fluorescence and characteristic color of fluorescence were used to evaluate the ability of each isolate to produce aflatoxins [32-35]. Aflatoxin diffusion areas shows a blue radiation around the isolate under UV light.
Mycotoxins Assays
Three different mycotoxins (Aflatoxin B1, Ochratoxin A and fumonisin B) were tested on smoked fish samples. Semi-quantitative immuno-chromatographic assay was used to detect Aflatoxin B1 (AFB1) and Fumonisin B (FB) while ELISA assay was used to detect Ochratoxin A (OTA). Briefly, the AFB1 assay was based on competitive inhibition of colloid gold immunochromatography principle. 2g of composite sample were grounded into centrifuge tube and 2 ml of 70% methanol-water solution mixture (V /V=7:3) were added to obtain a detection limit of 10 ppb. The mixture was shaked for 5 min and centrifuged at 4000 rpm for 5 min at room 25°C. Then 0.1 ml up-layer clear liquid was added to 0,4 ml of deionized water and mixed. 3 drops of this mixture were dropped on the sample collecting region of the test card. The negative result showed a red T line on the test card and positive result showed an invisible red T line on the test card.
Concerning the detection of OTA, 5 g of composite sample was introduced into 50 ml centrifuge tube with 10 ml of 70% methanol-water solution mixture (V/V=7 :3), Then shaked and centrifuged at 4000 rpm for 5 min to get supernatant. 1 ml supernatant was dried at water bath at 50°C. For the test procedure, 500 μl of sample buffer was added into centrifuge tube and vortexed until fully dissolve the dry residue on well and 100 μl of sample was dropped into sample region of card test the results were readed after 5 min. A negative result showed a T line on the test card and a positive result showed an invisible T line on the test card.
For FB test, 50 g of composite sample was grounded; 3 g were added into 3 ml of 80% methanol and mixed for 15 minutes at room temperature. After centrifugation for 10 minutes at 2000 rpm, 50 μl of the obtained supernatant were added to 150 μl of dilution buffer provided by the FB measurement ELISA kit (Euro Proxima) according to the manufacturer’s instruction.
Data Analysis
The frequencies and market distributions of fungal isolates were determined using proportion and rates. The results were presented in tables and figure. Mycotoxins dosage and humidity rate was assessed in triplicate.
Results
Smoked Fishes’ Identification
Smoked fish samples collected in different market of the city of Yaoundé belonged to 3 families namely, Claroteidae, Clupeidae and Scombridae. Claroteidae family was represented by one genus (Chrysichthys) and one specie (Chrysichthys sp). The Clupeidae family was represented by two genera (Ethmalosa and Sardinella) represented respectively by one specie each (Ethmalosa fimbriata and Sardinella maderensis). Scombridae family was represented by one genus (Scomberomorus) and one specie (Scomberomorus tritor).
All smoked fish samples species were collected in different markets according to their availability and their quantity (Table 1).
Smoked Fishes Specimen Species
Number of Specimens Collected by Markets
Total
Mokolo market
Central Market
Melen Market
Mfoundi Market
Mvog Betsi Market
Nsam Market
Ekounou Market
Etoudi Market
8th market
Essos Market
Ethmalosa fimbriata
40
40
20
40
20
20
20
20
20
20
260
Sardinella maderensis
40
40
20
40
20
20
20
20
20
20
260
Chrysichtys sp
40
20
20
40
20
20
20
20
20
20
240
Scromberomonus tritor
40
20
20
40
20
20
20
20
20
20
240
Totals
160
120
80
160
80
80
80
80
80
80
1000
Table 1: Distribution of smoked fish’s specimen collected from different markets.
Relative Humidity of Smoked Fish Samples
The average relative humidity rate of smoked fish samples (Table 2) was 7.70±2.9 % for Ethmalosa fimbriata, 8.60±3.6% for Sardinella maderensis, 14.60± 3.4% for Chrysichthys sp and 18.9± 4.3% for Scomberomonus tritor.
Fish Species
Relative Average Humidity
Rate (%)Limited Relative Humidity Value for Fungal Growth (%)
Ethmalosa fimbriata
7.70±2.9
12.0
Sardinella maderensis
8.60±3.6%
Chrysichtys sp
14.6±3.4
Scomberomonus tritor
18.9±4.3
Table 2: Relative Humidity rate of smoked fish samples species sold in Yaoundé markets.
Fungal Contamination of Smoked Fish Samples
18 composites samples (36%) amongst 50 composites samples were contaminated by fungal flora. Scomberomonus tritor was the most contaminated smoked fish sample with 12 composites contaminated samples (69,23%) followed by Chrysichtys sp 7 composites contaminated samples (60 %) and Sardinella maderensis 3 composites contaminated samples (25 %) (Table 3).
Fish Species
Number of
Specimen CollectedNumber of Composite Sample
Number of Composite Samples Contaminated by Fungus
Occurence of Fungal Contamination (%)
Ethmalosa fimbriata
300
15
0
0
Sardinella maderensis
240
12
3
25
Chrysichtys sp
200
10
6
60
Scomberomonus tritor
360
13
9
69,23
Total
1000
50
18
36
Table 3: Fungal contamination of smoked fish species sold in Yaoundé markets.
Fungi Identification
22 fungi isolates were obtained from 50 smoked fish composites samples. These isolates were grouped into 3 genera namely Aspergillus, Absidia and Penicillium base to their cultural characters (Figure 2). Aspergillus genera counted for 90% of isolates and was represented by 4 species namely Aspergillus flavus, Aspergillus niger, Aspergillus candidus and Aspergillus fumigatus, Absidia and Penicillium genera were represented respectively by one specie each (Absidia sp and Penicillium sp) (Figure 3).
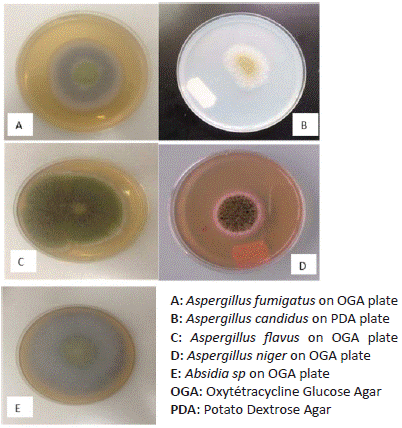
Figure 2: Cultural characters of fungi isolates on culture media.
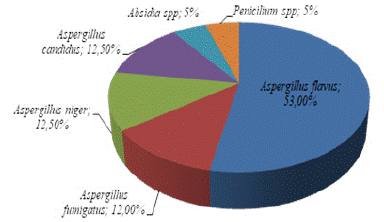
Figure 3: Distribution of Fungi species isolated from smoked fish sold in Yaoundé markets.
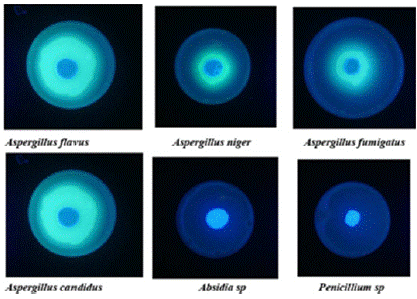
Figure 4: Aflatoxin producing isolates identification under UV light.
Distribution Fungi Species According to Smoked Fish Species
The distribution of fungi species according to fish species indicated that only 3 species of smoked fish were contaminated by molds (Table 4). Scomberomonus tritor was the most regulary infested smoked fish species followed by Chrysichtys sp and Sardinella maderensis. No fungal specie was isolated from Ethmalosa fimbriata.
Moulds Species Isolated
Smoked Fishes Species
Ethmalosa
Sardinella
Chrysichtys sp
Scomberomonus Tritor
Fimbriata
Maderensis
Aspergillus flavus
-
++
++++
++++++
Aspergillus candidus
-
-
+
++
Aspergillus niger
-
+
+
+
Aspergillus fumigatus
-
-
-
++
Absidia sp
-
-
-
+
Penicillium sp
-
-
+
-
Note: - : no isolate; + : one isolate ; ++ : two isolates; +++ : three isolates ; ++++ : four isolates ; +++++ : five isolates
Table 4: Distribution of fungi species according to smoked fish species.
Aflatoxin Producing Isolates Among Fungi Isolates
Only Aspergillus species isolates among the identified fungi species isolated from smoked fish were able to produce Aflatoxin. Amongst these, 9 isolates (75%) of Aspergillus flavus, 02 isolates (100%) of Aspergillus fumigatus, 3 isolates (100%) of Aspergillus candidus and 01 isolate (33,33%) of Aspergillus niger (33,33%) were able to produce Aflatoxin (Table 5).
Fungal Isolate
Number of Isolates Tested
Number of Isolates Positives to CEA Test
Percentage of Aflatoxin Producing Isolates (%)
Aspergillus flavus
12
9
75
Aspergillus niger
3
1
33,33
Aspergillus fumigatus
2
2
100
Aspergillus candidus
3
3
100
Absidia sp
1
0
0
Penicillium sp
1
0
0
Table 5: Percentage of Aflatoxin producing isolates amongst fungi species.
Mycotoxin Dosage in Smoked Fish Samples
Fifty composite aliquots representing all smoked fish species sample were analysed for the detection of mycotoxins. 17 aliquots (34%) were contaminated by mycotoxins (Figure 5). AFB1 was the main contaminant with 13(76 %) of aliquots followed by OTA 03 (18 %) and FB 1(6%).
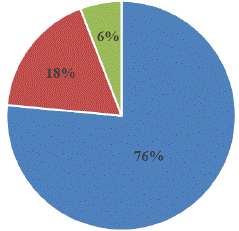
Figure 5: Distribution of mycotoxins detected in smoked fish samples sold in Yaoundé markets.
Discussion
In this study, thirty-six per cent (36%) of smoked fish sample were contaminated by fugal flora. This result which remains high is similar with Olajuyigbe et al. [36] study who obtained a contamination rate of 37% on retailed fishery products collected in Lagos markets. However, this result is different from Akwuobu et al. studies [37], who obtained an isolation rates ranging from 67.6% to 84.8%. This relative high contamination of smoked fish samples by fungal flora could result from lack of good hygiene practices and poor storage of smoked fish samples. It can also be due to relative humidity rate of fish during storage conditions. In fact, humidity is a factor favoring the proliferation of fungi on fish samples [38]. In other hand, the differences amongst isolations rates observed for the different smoked fish species could be explained by the differences in the nature of smoked fish specimen and the differences of processes associated with the smoked fish specimen. Scomberomonus tritor was the most contaminated fish sample with a fungal contamination rate of 69.23%. This result could be explained by the average humidity rate of this specimen. In fact, the humidity rate assessment of smoked fish samples showed that Scomberomonus tritor specimen has the highest humidity rate (18.9%) above the humidity rate limit for fungal growth (12%).
The present study revealed that fungal genera associated with contamination of smoke fish samples sold in Yaounde market were Aspergillus, Penicillum and Absidia (Figure 3). This result is similar with a few exceptions to that of Akwuobu et al. [37] studies who detected the genera Aspergillus, Penicillium, Mucor, Rhizopus, Absidia and Candida as the main contaminant species of smoke-dried fish sold in Makurdi market. According to Pitt and Hocking [39], Aspergillus is one of the most dominant contamination species found on dried food in tropical and subtropical regions [40]. The genera Aspergillus counted for 90% of fungi isolates with the proportions of 53% for Aspergillus flavus, 12.5% for Aspergillus niger and Aspergillus candidus and 12% for Aspergillus fumigatus. The dominance of Aspergillus sp as main contaminant of dried fish samples are an indication of its ubiquitous nature [41], its strong spore reproduction, its mycelium diffusion ability and its ability to adhere and survive in high protein nutrient sources such as dried fish [42]. Aspergillus species usually grows faster than Penicillium but takes longer to sporulate [43]. Penicillium sp was also isolated in significant numbers (18.18%) in our study. Similar finding has been documented by Rafli et al. [44], Essien et al. [45]. Essien et al. [45] for example have reported that Penicillium sp. constitutes the second most contaminants of dried fishes.
Assessment of the ability of fungal isolates to produce Aflatoxin showed that only Aspergillus spp isolates were able to produce Aflatoxin. This result is similar with that of Job et al. [46] study in Jos metropolis. In their study, they showed that among the mold’s isolates, only Aspergillus flavus strains presented aflatoxigenic producing potentials. In fact, Aspergillus familie’s members incuding Aspergillus flavus, A. parasiticus and Aspergillus ochraceus, Fusarium species [47] and Fusarium moniliforme [48] are recognized as the most common fungi mycotoxins producer. The detection of potentially aflatoxin-producing mold strains among of Aspergillus fumigatis, Aspergillus niger and Aspergillus candidus isolates in this study remains intriguing. But this result could be explained by an adaptation of these species to local conditions. But this requires further studies.
The detection of mycotoxins such as Aflatoxin B1, Ochratoxin A and Fumonisin B above acceptable limits in 34% of smoked fish sample indicates a dangerous sanitary risk associated with smoked fish consumption in Cameroon. Nowadays, food safety is an increasing preoccupation for consumers and of public health utilities [49].
In Cameroon, gastrointestinal cancers occurrence has raised over the last decade and among the risk factors associated with the increase of the prevalence of gastrointestinal tract cancers, contamination of foodstuffs by mycotoxins and particulary Aflatoxin B1 is often incriminated [50]. It is well documented that aflatoxin B1 is the most abundant genotoxic and carcinogenic mycotoxin [51], which could induce gastrointestinal and metabolic disturbances in contaminated foods such as smoke-dried fish [52]. Indeed, the detection of aflatoxins and ochratoxin A in smoked fish samples could also reflect the presence of Aspergillus and Penicillium species [37]. However, it would be interesting in future to measure these mycotoxins using a quantitative method such as HPLC-SM.
Conclusion
This preliminary study showed for the first time that smoked fish consumed in Yaoundé-Cameroon are heavily contaminated both by aflatoxin B1-producing fungi and by mycotoxins such as aflatoxin B1, Ochratoxin A and Fumonisin B, above acceptable limits.
Author Statements
Acknowledgements
The authors are grateful to the Head of Biochemistry department of the Faculty of sciences of the University of Douala for laboratories facilities and Pr Modeste SAMEZA for his contribution for Moulds identification.
Authors’ Contributions
Gwladys Ekwe Priso and Olivier Ziem are PhD students of the University of Douala and university of Yaoundé 1. This preliminary project did not receive external grant funding. The cost for all aspect of this research was covered by the funds from the research grant granted by the Cameroonian government to researchers of the state universities.
Francioli Koro koro conceived and designed the experiments. Olivier Ziem, Moïse Ntah, Gwladys Ekwe Priso, performed the experiments. Francioli Koro koro, Olivier Ziem, Moïse Ntah, Gwladys Ekwe Priso, Amandine Plidikoua, Moise Ntah A Ayong, Balbine Adande, Modeste Lambert SAMEZA analysed the data. Olivier Ziem and Gwladys Ekwe Priso wrote the first draft of the paper and designed figures. All authors provided critical input. Francioli Koro Koro and Francois-Xavier Etoa supervised the research, edited and approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethical Consideration
This study does not involve any human or animal testing.
References
- Food, Organization A. The state of world fisheries and aquaculture: contributing to food security and nutrition for all. Rome. 2016.
- Ngok E, Ndjamen D et D, J.v. Contribution économique et sociale de la pêche artisanale aux moyens d’existences durables et à la réduction de la pauvreté. 2005.
- Nunoo FKE, Kombat EO. Analysis of the microbiological quality of processed Engraulis encrasicolus and Sardinella aurita obtained from processing houses and retail markets in Accra and Tema, Ghana. World Journal of Fish and Marine Sciences. 2013; 5: 686–92.
- Adeyeye SAO, Oyewole OB, Obadina AO, Omemu AM. Microbiological assessment of smoked silver catfish (Chrysichthys nigrodigitatus. African Journal of Microbiology Research. 2015; 5: 1–9.
- Ineyougha ER, Orutugu LA, Izah SC. Assessment of microbial quality of smoked Trachurus trachurus sold in some markets of three South-States, Nigeria. International Journal of Food Research. 2015; 2: 16–23.
- Udochukwu U, Inetianbor J, Akaba SO, Omorotionmwan OF. Comparative assessment of the microbiological quality of smoked and fresh fish sold in Benin City and its public health impact on consumers. American Journal of Microbiological Research. 2016; 4: 1-4.
- Ayeloja AA, George FOA, Jimoh WA, Shittu MOAA, S.a. Microbial load on smoked fish commonly traded in Ibadan, Oyo State, Nigeria. Journal of Applied Science and Environmental Management. 2018; 22: 493–7.
- Likongwe MC, Kasapila W, Katundu M, Mpeketula P. Microbiological quality of traditional and improved kiln smoked catfish (Clarias gariepinus; Pisces; Clariidae) in Lake Chilwa Basin. Food Science and Nutrition. 2018; 7: 281–6.
- Wogu MD, Iyayi AD. Mycoflora of some smoked fish varieties in Benin City Nigeria. Ethiopian Journal of Environmental Studies and Management. 2011; 4: 36–8.
- Milicevic DR, Škrinjar M, Baltic T. Real and perceived risks for mycotoxin contamination in foods and feeds: Challenges for food safety control. 2010; 2: 572–592.
- Khiat N, Insaf. The confrontation of fungal strains isolated from the burnt forest floor of the Milia Region, thesis University of Constantine 1, Faculty of Nature and life Sciences. Department of Microbiology of Fungi. 2014; 61.
- Evelyne N, Tchuenchieu A, Mouafo HT, Fokou E, Medoua GN, De Saeger S, et al. Mycotoxin Contamination of Food and Associated Health Risk in Cameroon: A 25 years Review (1993-2018. European Journal of Nutrition & Food Safety. 2019; 9: 52–65.
- Ingenbleek L, Sulyok M, Adegboye A, Sètondji Hossou E, Zié Koné A, Oyedele AD, et al. Regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria Reveals the Presence of 164 Mycotoxins and Other Secondary Metabolites in Foods. Toxins. 2019; 11.
- Ostry V, Malir F, Toman JG, Y. Mycotoxins as human carcinogens The IARC Monographs classification. Mycotoxin Res. 2017; 33: 65–73.
- Rheeder JP, Marasas WFO, Thiel PG, Sydenham EW, Shephard GS, Schalkwyk DJ. Fusarium moniliforme and fumonisins in corn in human oesophageal cancer in Transkei. Phytopathology. 1992; 82: 353–7.
- Sun G, Wang S, Hu X, Su J, Huang T, Yu J, et al. Fumonisin B1 contamination of home-grown corn in high-risk areas for oesophageal and liver cancer in China. Food Additives and Contaminants. 2007; 24: 181–5.
- Ito Y, Peterson SW, Wicklow DT, Goto T. Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section. Flavi Mycological Research. 2001; 105: 233–9.
- Johnsson P, Lindblad M, Thim AM, Jonsson N, Vargas EA, Medeiros, et al. Growth of aflatoxigenic moulds and aflatoxin formation in Brazil nuts. World Mycotoxin Journal. 2008; 1: 127–37.
- Doster MA, Cotty PJ, Michailides TJ. Description of a Distinctive Aflatoxin Producing Strain of Aspergillus nomius that Produces Submerged Sclerotia. Mycopathology. 2009; 168: 193–201.
- Reddy KRN, Saritha P, Reddy CS, Muralidharan K. Aflatoxin B1 producing potential of Aspergillus flavus strains isolated from stored rice grains. African Journal of Biotechnology. 2009; 8: 3303–8.
- Klich MA, Pitt JI. Differentiation of Aspergillus flavus from A. parasiticus and other closely related species. Transactions of the British Mycological Society. 1988; 91: 99–108.
- W. PS, Ito Y, Horn BW, Goto T. Aspergillus bombycis, a new aflatoxigenic species and genetic variation in its sibling species. A nomius Mycology. 2001; 93: 689–703.
- Frisvad JC, Skouboe P, Samson RA. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-Omethylsterigmatocystin, Aspergillus rambellii sp. nov Syst Applied Microbiology. 2005; 28: 442–53.
- Kana JR, Gnonlonfin BBJ, Harvey J, Wainaina J, Wanjuki I, Skilton RA, et al. Assessment of aflatoxin contamination of maize, peanut meal and poultry feed mixtures from different agroecological zones in Cameroon. Toxins. 2013; 5: 884–94.
- Njobeh PB, Dutton MF, Koch SH, Chuturgoon AA, Stoev SD, Seifert KA. Contamination with storage fungi of human food from Cameroon. International Journal of Food Microbiology. 2009; 135: 193-198.
- Ngoko Z, Marasas WFO, Rheeder JP, Shephard GS, Zingfield MJ, Cardwell KF. Fungal infection and mycotoxin contamination of maize in the Humid forest and the western highlands of Cameroon. Phytoparasitica. 2001; 29: 352–60.
- ISO. Sampling procedures for inspection by attributes - Part 1: Sampling schemes indexed by acceptance quality limit (AQL) for lot-by-lot inspection. 2020.
- ISO. Microbiology of the food chain - Preparation of test samples, initial suspension and decimal dilutions for microbiological examination - Part 1: General rules for the preparation of the initial suspension and decimal dilutions.
- V08-059:2002. NF. Microbiology of food animal feeding stuffs - Enumeration of yeasts and moulds by colony-count technique at 25°C - Routine method.
- B. B, A. B, M. F, S. G, P. G, J.p. L, et al. Moisissures utiles et nuisibles, Importance industrielle. Ed., editor. Masson, Paris; 1990.
- B. PO-S, A. NP, S TA. Caractérisation de souches d’Aspergillus spp isolées des graines d’arachides cultivées au Burkina Faso, Afrique de l’Ouest. International Journal of Biological and Chemical Sciences. 2011; 5: 1232–49.
- Davis ND, Iyer SK, Diener UL. Improved method of screening for aflatoxin with a coconut agar medium. Journal of Applied and Environmental Microbiology. 1987; 53: 1593–5.
- T NM. Identification of mold species potentially producing mycotoxins in rice marketed in five provinces of the central region of Vietnam - study of conditions that can reduce mycotoxin production. Ecole doctorale de l’Institut National Polytechnique de Toulouse-France; 2007.
- Abdel Hadi A, Carter D, Magan N. Discrimination between aflatoxigenic and non-aflatoxigenic Aspergillus section Flavi strains from Egyptian peanuts using molecular and analytical techniques. World Mycotoxin Journal. 2011; 4: 69–77.
- Ezekiel CN, Adetunji MC, Atanda OO, Frisvad JC, Houbraken J, Samson RA. Phenotypic differentiation of species from Aspergillus section Flavi on neutral reddesiccated coconut agar. World Mycotoxin Journal. 2014; 7: 335–44.
- Olajuyigbe OO, Akande GR, Ezekiel CN, Ezekiel MO. Aflatoxigenic moulds and aflatoxin contamination of retailed fishery products in Lagos markets. Mycotoxicology. 2014; 1: 57–63.
- Akwuobu CA, Antiev WS, Ofukwu RA-P. Fungal Contaminants of Smoke-Dried Fish Sold in Open Markets in Makurdi, Benue State, North-Central Nigeria. Food and Nutrition Sciences. 2019; 10: 290–7.
- Demble C, Diarra O, Babana AH. Detection of aflatoxins in smoked fish (Clarias anguillaris) sold in the Bamako District. Journal of Microbiology and Experimentation. 2020; 8: 129–34.
- Pitt JI, Hocking AD. Aspergillus and related Teleomorphs. Fungi and Food Spoliage. 2009; 275–95.
- Kamil R, Darma Y, Andika S, Setyaningsih W, Anggrahini S, Agustinus P, et al. editors. Mold Contamination and aflatoxin B1 levels in salted fish commodities from traditional market in Yogyakarta and Surabaya, Indonesia. Malaysian Journal of Microbiology. 2018; 14: 691–8.
- Osibona AO, Ogunyebi OO, Samuel TO. Storage fungi and mycotoxins associated with stored smoked Catfish (Clarias gariepinus. Journal of Applied Sciences and Environment Management. 643–6.
- Deng Y, Wang Y, Deng Q, Sun L, Wang R, Ye L, et al. Fungal diversity and mycotoxin contamination in dried fish products in Zhanjiang market, China. Food control. 2021; 121: 107614.
- Rahayu ES, Sardjono S, A R. Pengenalan Jamur Benang pada Bahan Pangan (Introduction of mold in Food. In: Jamur Benang pada Bahan Pangan (Mold in Food. Yogyakarta: PT. Kanisius. 2014; 1–17.
- Rafli ZK, Damara DP, Andika S, Widiastuti S, Anggrahini S, Agustinus PR, et al. Mold contamination and aflatoxin B1 levels in salted fish commodities from traditional market in Yogyakarta and Surabaya, Indonesia. Malaysian Journal of Microbiology. 2018; 14: 691–8.
- Essien JP, Ekpo MA, Brooks AA. Mycotoxigenic and proteolytic potential of moulds associated with smoked shark fish (Chlamydoselachus anguincus. Journal of Applied Sciences and Environmental Management. 2005; 9: 53–7.
- Job MO, Agina SE, Dapiya HS. Occurrence of Aflatoxigenic Fungi in Smoke-dried Fish Sold in Jos Metropolis. British Microbiology Research Journal. 2016; 11: 1–7.
- Miller KY, Wu J, Miller BL. StuA is required for cell pattern formation in Aspergillus. Genes and Development. 1992; 6: 1770–82.
- Marasas WFO, Wehner FC, Rensburg SJ, Schalkwyk DJ. Mycoflora of corn produced in human esophageal cancer areas in Transkei, Southern Africa. Phytopathology. 1981; 71: 792–6.
- Naima S, Hider M, Doudah M, Errahmani MB, Hornick JL. In: effect of exogenous enzymes on performance of broiler chicken in Algeria. Agricultura. 2017.
- Ekwe Priso JGLF, Koro Koro F, Nda Mefo’o JP, Kojom Foko LP, Ziem O, Embolo Enyegue EL, et al. Epidemiological profile of gastrointestinal cancers in Douala, Littoral Region of Cameroon: A hospital-based retrospective study, 2016-2020. Asian Journal of Biology. 2022; 14: 45–55.
- Lahouar A. Mycotoxins and mycotoxigenic fungi in commercialized sorghum grains in Tunisia: Incidence and ecophysiological profiles. University of Lleida. Doctoral Thesis. 2016.
- Bennett J, Klich M. Mycotoxins. Clinical Microbiology Reviews. 2003; 16: 497–516.